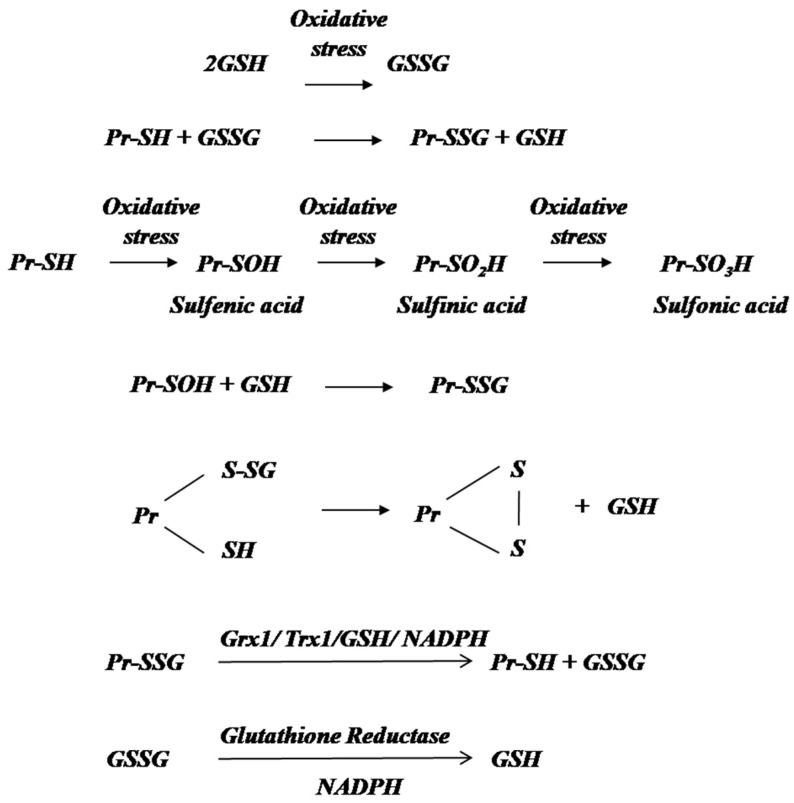
Figure 1.
Protein thiol modification during oxidative stress. During oxidative stress, GSH is oxidized to GSSG, which promotes the oxidative modification of protein thiols (Pr-SH) to protein glutathione mixed disulfides (Pr-SSG). Protein thiols can be directly oxidized to organosulfur oxoacids sulfenic (Pr-SOH), sulfinic (Pr-SO2H) and sulfonic acid (Pr-So3H). Sulfenic acid can be glutathionylated to prevent its further oxidation to irreversible sulfonic acids. Pr-SSG can undergo further modifications to form protein mixed disulfides. Pr-SSGs are reduced back to protein thiols by Grx1 or Trx1 utilizing the reducing equivalent of NADPH. GSSG formed in this reaction is effectively reduced to GSH by glutathione reductase using the reducing equivalent of NADPH.