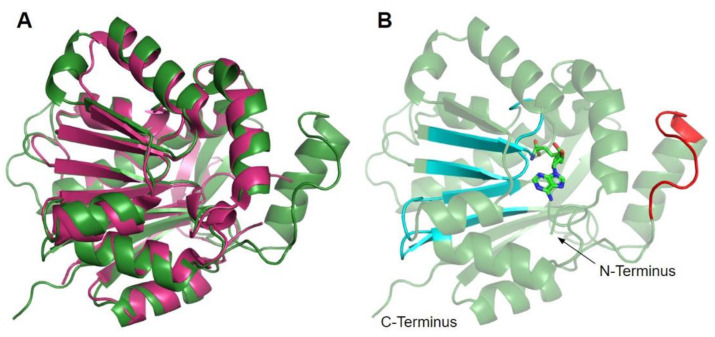
Figure 5.
Alignment of AlphaFold-generated model of human COQ3 with existing E. coli UbiG crystal structure. (A) Superimposition of the human COQ3 model (residues 91–336, shown in dark green), adapted with permission from [39,54], and the crystal structure of AdoHcy-bound E. coli UbiG (shown in magenta, PDB: 5DPM). The N- and C-termini of the model were omitted due to low confidence. Note that this and subsequent figures about COQ3 were generated using PyMOL, which classifies a short-coil region as a helix (top left of figure). This gives a total of nine helices as opposed to eight rendered in Figure 4. (B) Human COQ3 model with conserved methyltransferase motifs I–III and post-I highlighted in cyan and the hydrophobic region in red. AdoMet (shown in light green) was modeled via structural alignment with PDB 5DPM using PyMOL.