Figure 3.
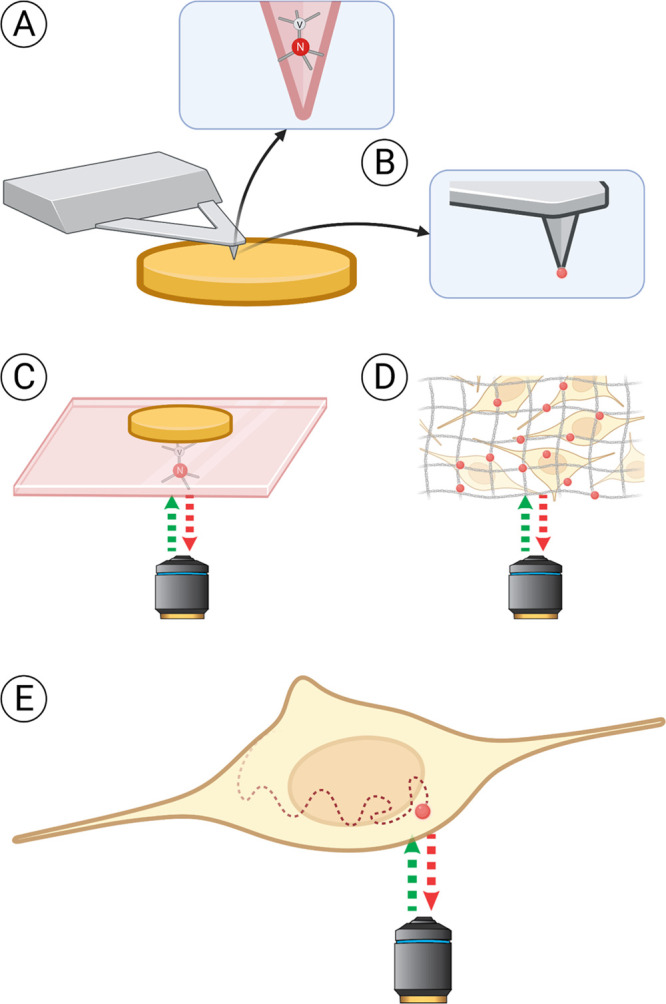
Different ways to conduct relaxometry experiments. The sample is shown in yellow, the (nano)diamond hosting NV centers in red. Three main approaches can be defined. In the first case (A, B), the sample is scanned with a NV-containing probe, similarly to atomic force microscopy. The scanning tip can either be made of diamond hosting NV centers (A) or have a nanodiamond attached to it (B). The second approach (C, D) relies on obtaining information from a stationary diamond placed close to the sample. Most commonly, bulk diamond plates are used for that (C). Nanodiamonds immobilized on a supporting surface or embedded in polymer matrices offer a cheaper alternative. They can be measured through a cover glass, in a microfluidic chip, or diluted in solution in high concentrations. Also, they offer shorter measurement times than bulk diamond plates due to shorter T1 time. However, with nanodiamonds there is less control over the orientation of the NV centers and the defect properties are usually worse (D). In the third approach (E), individual nanodiamonds diffusing in the sample (chemical solution or a live cell) are tracked during the experiment, allowing one to map the spatial differences or to choose a specific position for the measurements. Nevertheless, direct measurement of single nanodiamonds in solution without immobilization is challenging. In cells, FNDs are transported/diffuse relatively slowly or can be anchored to specific locations (e.g., mitochondrial membrane, cell membrane).1,3
