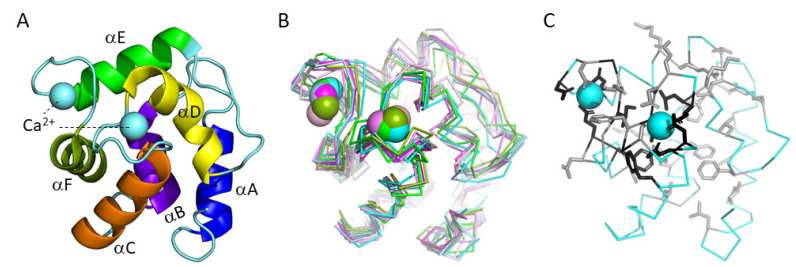
Figure 1.
Parvalbumins have six α-helices A-to-F and two “EF-hand” domains for binding Ca2+ ions (indicated as spheres). (A) The structure, in cartoon format, of common carp pvalb4_(Chr.A3) (PDB accession 4CPV) [10], which was the first parvalbumin of which the structure was elucidated [11]. Different α-helices are in different colors. (B) Superimposition of various parvalbumin structures, in ribbon format, reveals a common structure. Light pink, human α-parvalbumin (1RK9); pink, pike pvalb7 (2PAS); magenta, spotless smooth-hound shark SPV-I α-parvalbumin (5ZGM); green, human oncomodulin (1TTX); splitpea green, chicken CPV3-oncomodulin (2KYF); soft purple, chicken ATH (3FS7); cyan, Atlantic cod pvalb2 (2MBX); green cyan, pike pvalb3 (1PVB); aquamarine, common carp pvalb4_(Chr.A3) (4CPV); light teal, spotless smooth-hound shark SPV-II (5ZH6). (C) Most of the conserved EF-hand family residues belong to the Ca2+ binding regions (see also Figure 5). The structure, in ribbon format, of common carp pvalb4_(Chr.A3) (PDB accession 4CPV), shows in black those residues that are well conserved throughout EF-hand domain family molecules and in gray other residues that are well conserved throughout parvalbumins; the sidechains of these residues are shown in sticks format.