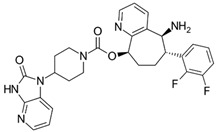
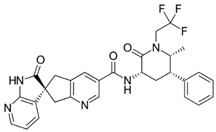
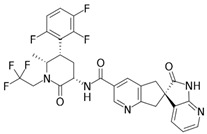
Table 1.
Comparative analyses of pharmacokinetics, dosage, and formulations of rimegepant, ubrogepant, and atogepant.
| Drugs | Rimegepant | Ubrogepant | Atogepant |
|---|---|---|---|
| Other names | BHV-3000, BMS-927711 | MK-1602 | AGN-241689, MK-8031 |
| Trade name | Nurtec®, Nurtec ODT®, Vydura® | Ubrelvy® | Qulipta® |
| Marketed | Biohaven Pharmaceuticals, Pfizer | Allergan Pharmaceuticals | Allergan Pharmaceuticals |
| FDA approval | February 2020 (acute); May 2021 (prevention) | December 2019 | September 2021 |
| Molecule |
|
|
|
| Indication | Acute treatment of migraine with or without aura in adults. Preventive treatment of episodic migraine in adults | Acute treatment of migraine with or without aura in adults | Preventive treatment of episodic migraine in adults |
| Dose | 75 mg PO as needed. Not to exceed 75 mg/ 24 h. Safety of >18 doses/30 days has not been established | 50 or 100 mg PO as needed. If needed, may take second dose at least 2 hrs after initial dose. Not to exceed 200 mg/24 h. Safety of treating >8 migraines/30 days has not been established | 10, 30, or 60 mg PO |
| Dose adjustments | Renal impairment (CrCl < 15 mL/min); hepatic impairment (Child–Pugh > C); drug interactions | Renal impairment (CrCl < 30 mL/min); hepatic impairment (Child–Pugh ≥ C); drug interactions | Renal impairment (CrCl < 30 mL/min); hepatic impairment (Child–Pugh ≥ C); drug interactions |
| Route of administration | Oral | Oral | Oral |
| Contraindications | History of hypersensitivity | Concomitant use with strong CYP3A4 inhibitors | None |
| Adverse effects | Nausea (2%), hypersensitivity (including delayed) | Nausea (2–4%), somnolence (2–3%), dry mouth (2%) | Nausea (5–9%), constipation (6%), somnolence (4–6%), elevated AST/ALT (1%) |
| Pregnancy and lactation | Data not available | Data not available | Data not available |
| Pediatric | Data not available | Data not available | Data not available |
| Considerations | Orally disintegrating tablet. It can be swallowed without additional liquid | High-fat meal delayed plasma concentration | High-fat meal effect was not significant. |
