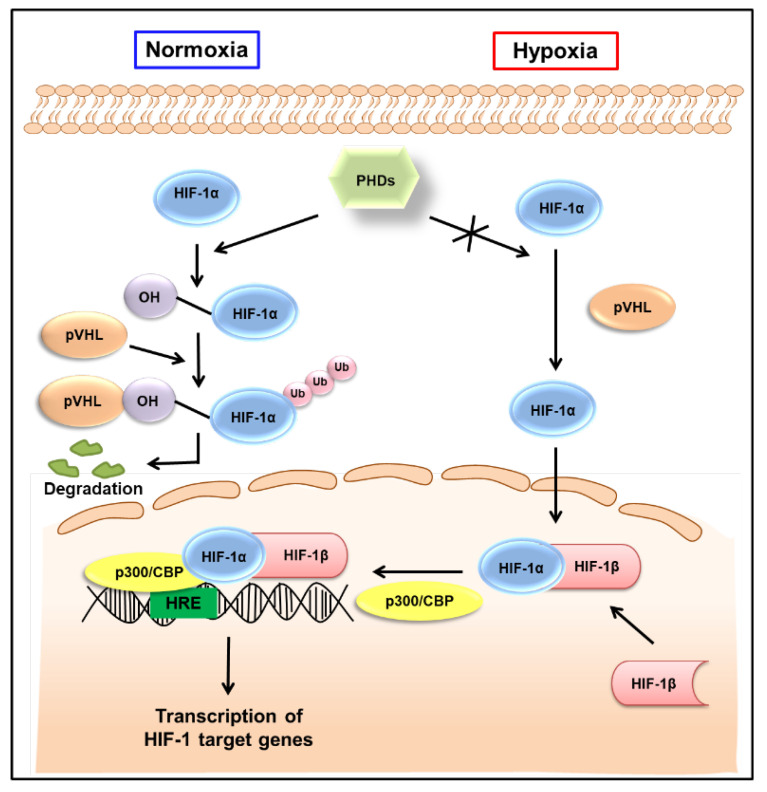
Figure 1.
Regulation of HIF-1 activity in normoxic and hypoxic conditions. Under normoxic conditions, HIF-1α is hydroxylated on prolyl residues by PHDs, allowing pVHL to recognize and interact with HIF-1α, eventually leading to the degradation of HIF-1α. By contrast, HIF-1α is stable in hypoxic conditions. It is translocated to the nucleus, where, together with HIF-1β, it binds to hypoxia-responsive elements (HREs) and exerts its transcriptional activity. HIF-1—hypoxia-inducible factor-1; PHDs—prolyl hydroxylase domains; pVHL—von Hippel−Lindau tumor suppressor protein; Ub—ubiquitination; CBP—cyclic adenosine monophosphate response element binding protein.