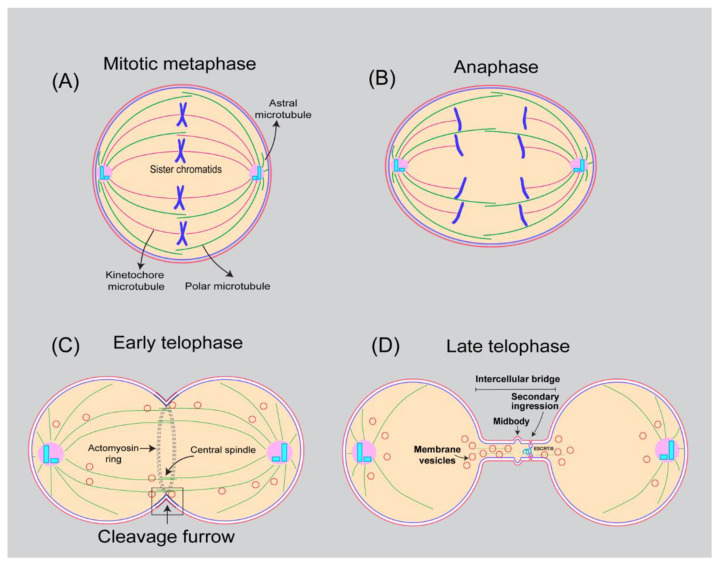
Figure 1.
Schematic representation of mitotic cells undergoing division. (A) At metaphase, cells acquire round shape, chromosomes attached to kinetochore microtubules become aligned at the equatorial plane. (B) At anaphase, cells elongate, sister chromatids separate, and the daughter chromosomes move towards the poles. (C) Once daughter chromosomes reach poles and form two nuclei, the cytoplasm between the two nuclei is divided and physically separated by a process known as cytokinesis. During early stages of telophase, the mitotic spindle determines the site of cleavage furrow, by recruiting two master regulators Centralspindlin complex and Chromosomal Passenger Complex (CPC). These multisubunit complexes activate downstream signaling proteins including Rho-GEF (Ect-2), Rho GTPase and cytoskeletal regulators to promote assembly of actomyosin contractile ring (composed of unbranched actin and myosin-II) positioned midway between segregated chromosomes. Plasma membrane at the cytokinetic furrow is anchored to the contractile ring via Anillin and Septins and anionic phospholipid PI(4,5)P2. Constriction of the ring with concomitant membrane addition via both endocytosis and forward trafficking, generates the cleavage furrow. (D) Cleavage furrow ingression and stabilization results in the formation of intercellular bridge with an electron dense structure in the middle called midbody. The final steps of cytokinesis known as abscission involve endocytic trafficking, clearance of PIP2 on furrow membrane, microtubule severing, F-actin clearance, fusion of endosomes with the plasma membrane leading to secondary ingression. In a final step, there is assembly of the endosomal sorting complex required for transport (ESCRT III) helical filaments downstream of Cep55 and ALIX-TSG101 activation, resulting in membrane cutting that will physically separate the cytoplasm between two daughter cells.