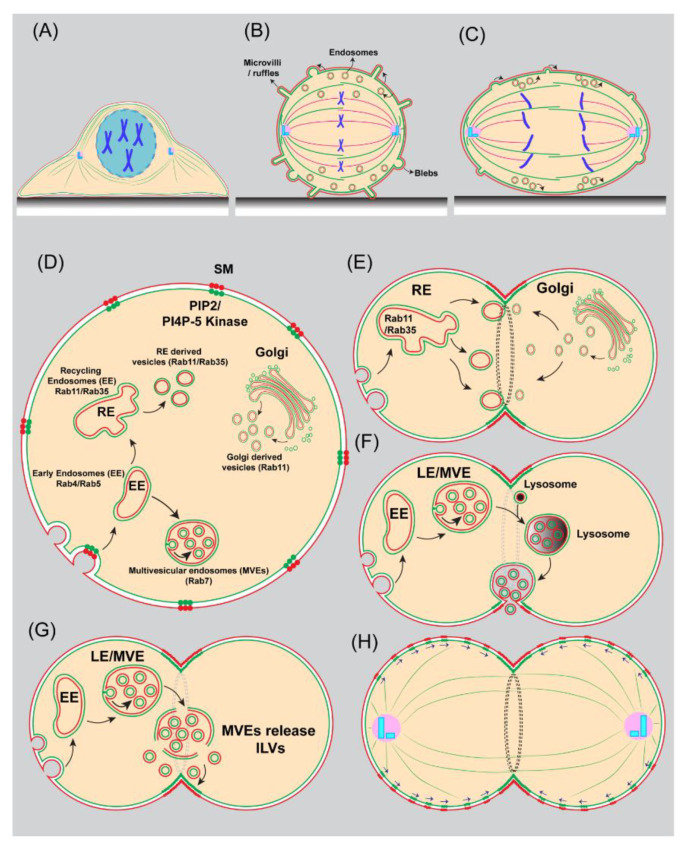
Figure 4.
Mechanisms for membrane traffic and lipid polarization during cytokinesis. (A–C) Regulation of plasma membrane area in a dividing mammalian cell. (A) At the interphase, cultured, adherent mammalian cells appear flat; (B) at the onset of mitosis, cells lose their attachment to the substrate and begin to round up and reduce their surface area. To accommodate this change in surface area, the plasma membrane folds into blebs, microvilli, and ruffles and becomes endocytosed. (C) During anaphase, cell surface area increases, which correlates with the fusion of recycling endosomes with the plasma membrane and the expansion of membrane projections. (D) An eukaryotic cell in prophase showing endomembrane compartments, including early endosomes (EE), recycling endosomes (RE), multivesicular endosomes (MVEs), the Golgi apparatus, and vesicles derived from these compartments. The plasma membrane bilayer is shown with an outer leaflet (red) and an inner leaflet (green). Circular dots on the plasma membrane correspond to lipid domains, including sphingomyelin-rich domains (red dots) and Phosphatidylinositol-4, 5-bisphosphate (PIP2) and its biosynthetic enzyme PI(4)P 5-kinase (green dots). (E) A diagram depicting membrane trafficking from recycling endosomes and the Golgi apparatus to the cleavage furrow. (F) Cartoon showing membrane addition via lysosome exocytosis at the cleavage furrow in mammalian cells. Late endosomes (LE), Multivesicular endosomes (MVE). (G) Schematic representation of membrane trafficking via multivesicular endosomes that release their intraluminal vesicles in the cytosol near cleavage furrow in Drosophila spermatocytes. (H) Illustration of lateral movement of transbilayer-coupled lipid domains towards the cleavage furrow via a cortical flow mechanism.