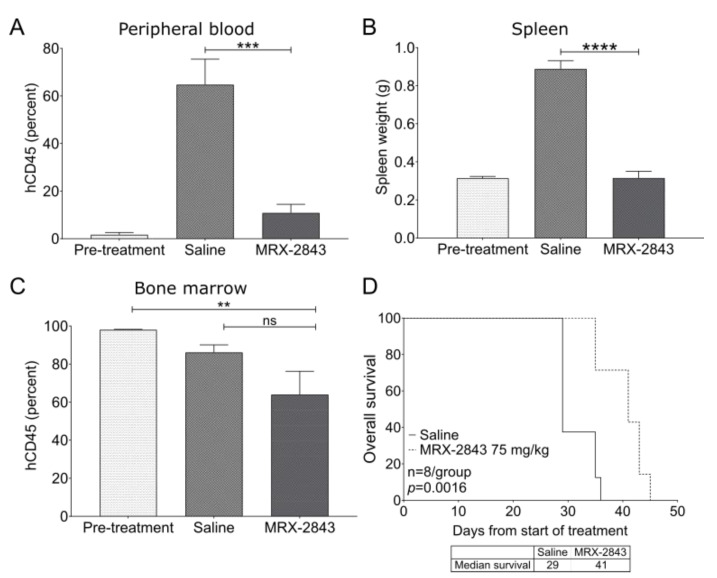
Figure 6.
MRX-2843 monotherapy promotes leukemia clearance in peripheral blood and spleen, and prolongs survival in a patient-derived ETP-ALL xenograft model. (A–D) NSGS mice were inoculated with xenograft-passaged cells from a patient with ETP-ALL (ETP0068TJ) and treatment was initiated 36 days later. (A–D) A cohort of mice were harvested prior to the start of treatment (pre-treatment). The remaining mice were treated once daily with 75 mg/kg MRX-2843 or saline vehicle. (A–C) After treatment for 29 days, peripheral blood (A), spleen (B), and bone marrow (C) were collected and leukemic blasts (hCD45+) were detected by flow cytometry. Spleen weight was used as a surrogate for splenic disease burden. Mean values ± SEM are shown (** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001, one-way ANOVA, n = 6). (D) Survival was monitored in the remaining mice and was significantly prolonged in mice treated with MRX-2843 (p = 0.0016, log-rank test, n = 8). Abbreviations: ns—not significant.