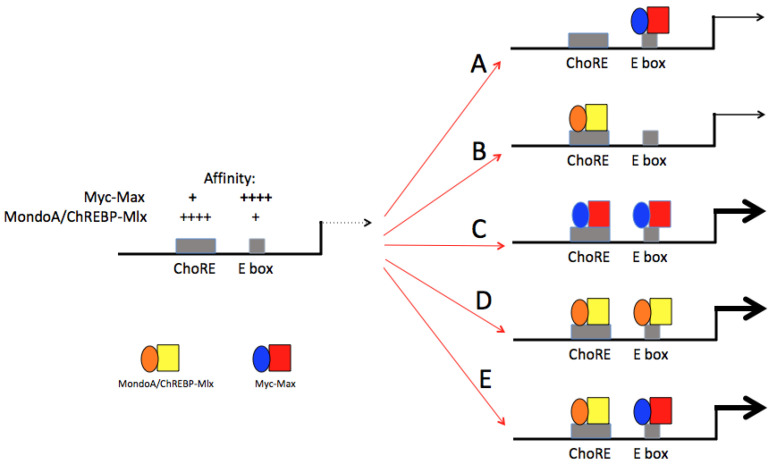
Figure 2.
Co-regulation of common target genes by Myc and Mlx Network members. Possible transcriptional scenarios in response to binding by Myc and/or Mlx Network members. On the left, a hypothetical gene bearing an E box that is either of high- or low-affinity for Myc (++++ and +, respectively) (small gray box). It also contains a ChoRE of similar affinities for MondoA/ ChREBP-Mlx (large gray box). The gene is expressed at a low basal level in the absence of Myc and MondoA/ChREBP as might occur in quiescent cells maintained in low glucose. (A). In a low glucose environment, Myc may be induced in response to a normal proliferative signal. Myc-Max heterodimers bind to the high-affinity E box but not to the ChoRE and activate transcription in a glucose-independent manner. (B). In quiescent cells in a high glucose environment, MondoA/ChREBP-Mlx heterodimers enter the nucleus, bind to the high-affinity ChoRE but not to the E-box and activate transcription in a glucose-dependent manner. (C). In tumor cells that express excessively high Myc levels, Myc binds to both the high affinity E box and the low-affinity ChoRE. In the latter case, Myc-Max levels may be sufficiently high so as to exclude MondoA/ChREBP-Mlx binding entirely despite their otherwise high affinities for this site and particularly if they are expressed at low levels. Gene expression is now again glucose-independent. (D). In quiescent cells in which MondoA and/or ChREBP are particularly high, they may bind to both high-affinity ChoREs and low-affinity E boxes and regulate gene expression in a manner that is now glucose-dependent and Myc-independent. (E). In response to a normal proliferative stimulus and high-glucose, the gene is induced to a high level as a result of Myc-Max and MondoA/ChREBP-Mlx heterodimers each binding their respective high-affinity sites.