Figure 7. Cellular model of aldosterone-regulated electrogenic K+ secretion in the distal colon.
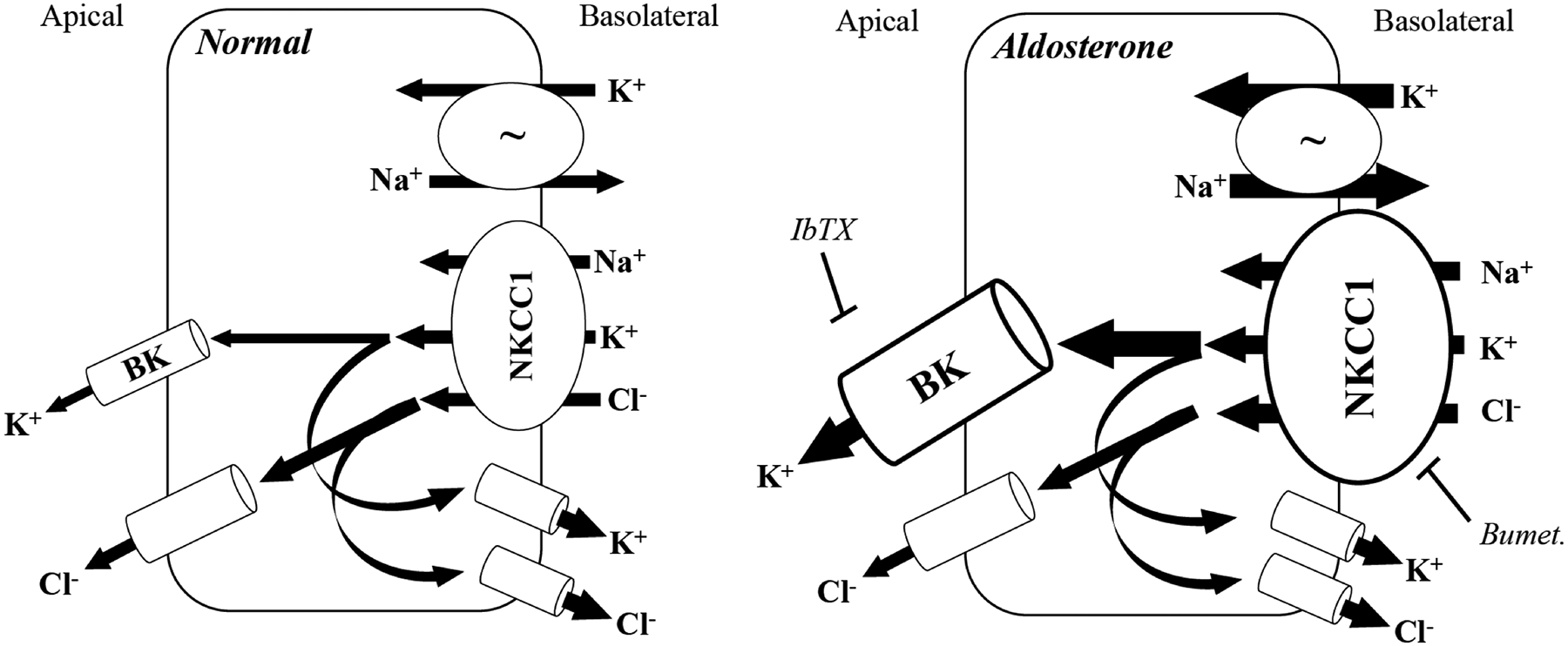
Normal distal colon secretes both K+ and Cl− across the apical membrane. Na+, K+ ATPase (~) activity drives overall transport by establishing the Na+ gradient that allows basolateral uptake of K+ and Cl− via NKCC1. Apical membrane secretion of K+ and Cl− is supported by basolateral exit of Cl− and K+ ions, respectively, to maintain the necessary electrochemical gradient. Multiple basolateral K+ and Cl− channels may be involved in this process, including intermediate conductance (IK) and voltage-gated (KV7.1) K+ channels, as well as CLC-2 Cl− channels. Aldosterone stimulates electrogenic K+ secretion by increasing basolateral Na+, K+ ATPase activity (represented by enlarged arrows) and increasing the expression and activity of BK channels and NKCC1 co-transporter (represented by enlarged image objects and arrows, respectively). Electrogenic K+ secretion is inhibited by either IbTX (apical membrane BK channels) or bumetanide (basolateral NKCC1). To support the aldosterone-induced sustained K+ secretion at the apical membrane, basolateral Cl− exit also must presumably be enhanced, although the identity of the channel(s) involved remains undetermined. Despite the increase in NKCC1 expression and activity, electrogenic Cl− secretion is not enhanced by aldosterone, suggesting that the regulation of NKCC1 by aldosterone is specific to the K+ transport pathway.
