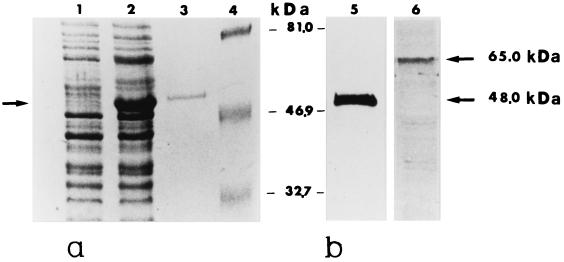
FIG. 1.
(a) Expression, purification, and immunoblots of the recombinant ScMp65 protein. Cells of E. coli M15 (pUHA1 and pRLV126) either noninduced (lane 1) or IPTG induced (lane 2) were extracted and subjected to SDS-PAGE, together with the recombinant ScMp65 (nickel-nitrilotriacetic acid [Ni-NTA] affinity chromatography purified) (lane 3). The gels were stained with Coomassie blue. Lane 4 shows the molecular mass standards (in kilodaltons). (b) Immunoblots of ScMp65 (lane 5) or of a cell wall extract of C. albicans (lane 6) with a mouse antiserum raised against the purified ScMp65. A total of 1 μg of the purified protein or 10 μg of cell wall extract was run, electrotransferred to nitrocellulose membranes, and immunoblotted with the anti-ScMp65 serum (dilution, 1:800). The reaction was developed with alkaline phosphatase-conjugated anti-mouse (1:5,000) immunoglobulin G. The arrows to the right side of lane 6 point to the apparent molecular mass of the ScMp65 in lane 5 and its correspondent of the natural Mp65 of C. albicans in lane 6.