Abstract
Background: Immunocompromised patients, including those with hematological malignancies, are at a high risk of developing severe coronavirus disease 2019 (COVID-19) complications. Currently, there is a limited number of systematic reviews into the efficacy of convalescent plasma therapy (CPT) use in the treatment of COVID-19 patients with hematological malignancies. Therefore, the aim of this review was to systematically appraise the current evidence for the clinical benefits of this therapy in COVID-19 patients with hematological malignancies. Methods: A comprehensive search was conducted up to April 2022, using four databases: PubMed, Web of Science, Science Direct, and Scopus. Two reviewers independently assessed the quality of the included studies. Data collection analysis was performed using Microsoft Excel 365 and GraphPad Prism software. Results: 18 studies met the inclusion criteria; these records included 258 COVID-19 patients who had hematological malignancies and were treated with CPT. The main findings from the reviewed data suggest that CPT may be associated with improved clinical outcomes, including (a) higher survival rate, (b) improved SARS-CoV-2 clearance and presence of detectable anti-SARS-CoV-2 antibodies post CP transfusion, and (c) improved hospital discharge time and recovery after 1 month of CPT. Furthermore, treatment with convalescent plasma was not associated with the development of adverse events. Conclusions: CPT appears to be an effective supportive therapeutic option for hematological malignancy patients infected with COVID-19. To our knowledge, this is one of the first systematic reviews of the clinical benefits of CPT in COVID-19 patients with hematological malignancies.
Keywords: convalescent plasma, COVID-19, SARS-CoV-2, hematological malignancy
1. Introduction
Since the first case report in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of the coronavirus disease 2019 (COVID-19), has posed a significant challenge worldwide [1]. The clinical manifestation of COVID-19 ranges from having no signs or symptoms (asymptomatic) to severe complications that include thrombosis, septic shock, acute respiratory distress syndrome (ARDS), and cardiac failure [2]. Immunocompromised patients and cancer patients are among those who are at a high risk of a severe and prolonged disease course [3,4]. Hematological malignancies are heterogeneous blood cancers categorized according to the sites of origin—blood (leukemias), lymph nodes (lymphomas-Hodgkin and non-Hodgkin), or bone (myelomas) [5]. Patients with hematological malignancies represent a distinctive subset of those vulnerable to COVID-19 and were shown to be frequently associated with high mortality and COVID-19 complications [6]. Due to the underlying disease and cancer treatment, the immune system in these patients becomes impaired, thus making them immunodeficient and prone to infection and severe disease [2].
Convalescent plasma therapy (CPT) is a form of passive immunity where plasma enriched with specific and non-specific humoral innate immunity factors is collected from recovered patients, processed, and transfused into other patients [7]. During viral infection, the antibodies are key for virus opsonization and neutralization, in addition to the activation of complement and mediation of antibody-dependent cellular cytotoxicity. This type of treatment has been previously used to treat other infectious diseases such as Ebola, SARS, Middle East respiratory syncytial virus (MERS), and influenza [2]. Currently, COVID-19 has very limited treatment options, with isolation and supportive care being the major ones [8]. Some natural fruit and plant bioactive compounds have been shown to inhibit protease activity in SARS-CoV-2 [9]. In August 2020, the United States Food and Drug Administration (US FDA) issued an emergency use authorization (EUA) for COVID-19 convalescent plasma for the treatment of hospitalized patients with COVID-19 [10]. As a result, numerous trials have been conducted to assess the effectiveness of CPT in different COVID-19 patient cohorts including those with different disease severity and co-morbidities. The effectiveness of COVID-19 CPT in immunocompromised cancer patients, particularly those with hematological malignancies, has not been systematically reviewed yet. Therefore, we conducted a systematic review of the effectiveness of CPT to treat COVID-19 patients with hematological malignancies.
2. Methods
The study’s focus was on hematological malignancy patients (in remission and progression) with confirmed PCR COVID-19 infection. The intervention was CPT from previously infected COVID-19 patients. The control group had no CPT intervention, and studies with no control group were also included. The primary goal of this study was improvement in clinical outcomes, measured by survival outcomes and mortality, development of adverse events, and hospital discharge. The secondary goal was measured by viral clearance, defined as two consecutive negative RT-PCR test results 24 h apart and/or a decrease in RNAemia. SARS-CoV-2 RNAemia was measured using droplet-based digital RT-PCR (ddPCR) technology. We followed PRISMA guidelines in this search. The search was conducted up to June 2022 by two authors, using four major databases: PubMed, Web of Science, ScienceDirect, and Scopus. The following terms were used: ‘COVID-19’ or ‘SARS-CoV-2’ or Coronavirus’ AND ‘convalescent plasma’ or ‘convalescent plasma therapy’. All articles were extracted and assessed by two independent authors to ensure the quality of the research. All articles were exported to Endnote X9 and all duplicates were removed.
2.1. Inclusion and Exclusion Criteria
Articles were screened by title and abstracts. The following inclusion criteria were used for eligibility: (1) reported in English, (2) clinical trials including randomized and controlled clinical trials, and (3) prospective and retrospective comparative cohort studies, case-control studies, cross-sectional studies, case series, and case reports. Studies were excluded if they did not meet the inclusion criteria. In addition, all letters, editorials, systematic reviews, narrative reviews, abstracts, and non-full-text articles were excluded.
2.2. Quality Assessment and Risk of Bias Assessment
The risk of bias for all eligible observational studies was assessed according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines by two authors, and if there was any discrepancy, it was resolved by consulting a senior author [11]. The risk was evaluated using a previously published question tool [12], which asked questions regarding (1) selection criteria of patients—namely, whether all the patients met the inclusion criteria; (2) adequate ascertainment of exposure and the outcome; (3) causality—namely, whether follow-up was long enough for outcomes to occur; and (4) reporting—namely, whether the case(s) were described with sufficient detail to allow other investigators to replicate the research or to allow practitioners to make inferences. All studies were scored for each question as: yes (2 stars), partial (1 star), and no (0 stars). An overall risk of bias was independently assigned to each eligible study by two authors. No studies rated below 5 were included in the systematic review (Table 1).
Table 1.
Risk of Bias Assessment.
| Author | Selection Criteria of Patients? * | Adequate Ascertainment Regarding the Exposure and the Outcome? * | Causality? * | Reporting? * | Total Score |
|---|---|---|---|---|---|
| Shankar et al. [7] | 2 | 2 | 2 | 2 | 8 |
| Szwebel et al. [13] | 2 | 2 | 2 | 2 | 8 |
| Wright et al. [14] | 2 | 2 | 2 | 2 | 8 |
| Tremblay et al. [8] | 2 | 2 | 2 | 2 | 8 |
| Thompson et al. [15] | 2 | 2 | 2 | 2 | 8 |
| Luetkens et al. [16] | 2 | 2 | 2 | 2 | 8 |
| Moore et al. [17] | 2 | 2 | 2 | 2 | 8 |
| Malsy et al. [18] | 2 | 2 | 2 | 2 | 8 |
| Rnjak et al. [2] | 2 | 2 | 2 | 2 | 8 |
| Balashov et al. [19] | 2 | 2 | 2 | 2 | 8 |
| Biernat et al. [20] | 2 | 2 | 2 | 2 | 8 |
| Çınar et al. [21] | 2 | 2 | 2 | 2 | 8 |
| Dell’Isola et al. [22] | 2 | 2 | 2 | 2 | 8 |
| Ferrari et al. [23] | 2 | 2 | 2 | 2 | 8 |
| Hueso et al. [24] | 2 | 2 | 2 | 2 | 8 |
| Jeyaraman et al. [25] | 2 | 2 | 2 | 2 | 8 |
| Karatas et al. [26] | 2 | 2 | 2 | 2 | 8 |
| Oliva et al. [27] | 2 | 2 | 2 | 2 | 8 |
* Yes (2 stars), partial (1 star), and no (0 star).
2.3. Data Extraction and Synthesis
The following clinical and laboratory variables were extracted: country, gender, age, type of hematological malignancy, cancer treatment, number of patients with CPT intervention, number of controls without CPT, and other treatments for COVID-19. Clinical outcomes including survival rate, adverse effects of CPT, and the titer of antibodies for donor and patient were recorded. Data analysis was qualitative: the collected data were interpreted based on the presence or absence of the clinical outcomes. The findings were collected using Microsoft Excel sheets.
2.4. Statistical Analysis
The clinical data that were extracted from the articles and assessed included survival, clinical outcome, viral clearance, seropositivity to SARS-CoV-2 antibodies, hospital discharge, CP dose, recovery period, development of adverse events to CPT, and companion treatments. The data analysis was only qualitative, and the collected data were interpreted based on the presence or absence of the clinical outcome. A meta-analysis was performed using GraphPad Prism Version 9.0 software to calculate the odds ratio and generate the forestplot. Dichotomous outcomes are presented as odds ration (OR) with 95% confidence intervals (CIs).
3. Results
3.1. Search Findings
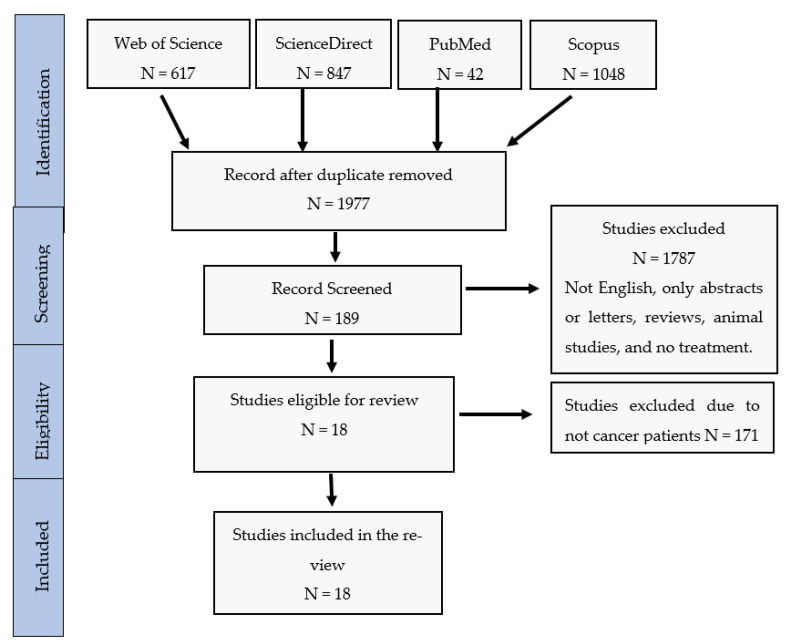
The outcome of the database search is described in a PRISMA flow chart (Figure 1). The search process yielded 2553 records, of which 578 articles were identified as duplicates and removed. Following title and abstract screening of the remaining 1975 articles, 1787 were excluded from the analysis due to one or more of the following reasons: (1) the article was published in a language other than English, (2) it was not an original research article, (3) it was an animal-based study, (4) the full-text was not available, or (5) the CPT was not used as a COVID-19 treatment. From the 188 full texts screened, 171 studies were excluded because they were not conducted on patients with hematological malignancies. Seventeen studies met the inclusion criteria and were used to perform this systematic review. We identified 1103 patients, of which 258 patients had one or more hematological malignancy, were diagnosed with COVID-19, and were treated with CPT, whereas 845 patients were included in the control group and were provided with standard care in two of the identified studies [15,20].
Figure 1.
PRISMA Flow chart of study selection.
3.2. Study Characteristics and Patients’ Demographics
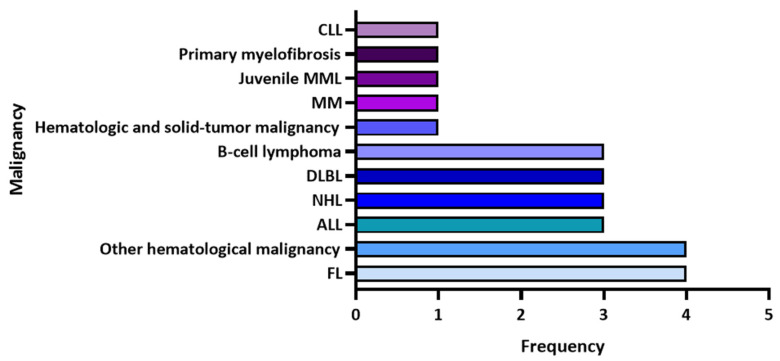
Among the 17 selected articles, 13 were case reports or case series, two were retrospective cohort studies, and two were observational multicenter studies (Table 2). The reported hematological malignancies were mainly follicular lymphoma (n = 4), chronic lymphocytic leukemia ((n = 3), non-Hodgkin’s lymphoma (n = 3), diffused large B-cell lymphoma (n = 3), B-cell lymphoma (n = 3), and unspecified hematological malignancies (n = 4), (Figure 2). The range of patients’ ages was from 9 months to 72 years; the majority were older than 50 years while only three children were included (9 months and 4 and 5 years). A higher proportion of male patients were included in the studies compared to females (8:3). Ten patients had a history of receiving a stem cell transplant, of which seven were autologous transplants.
Table 2.
Summary of included studies on CPT for COVID-19 patients with hematological malignancies.
| Author | Year | Country | Malignancy | Study Design | Sample Sizes CPT/Non-CPT |
Age Median (Range) | Gender | Transplant |
|---|---|---|---|---|---|---|---|---|
| Shankar et al. [7] | 2021 | United States | ALL | Case report | 1/0 | 4 years | F | |
| Szwebel et al. [13] | 2021 | France | HIV and cancer B-cell lymphoma | Case report | 1/0 | NA | M | ASCT |
| Wright et al. [14] | 2021 | United States | FL | Case report | 1/0 | 54 years | M | |
| Tremblay et al. [8] | 2020 | United States | Hematologic and solid cancer | Case series | 24/0 | 69 years (31–88) | 41.7% F, 58.3% M | |
| Thompson et al. [15] | 2021 | Unites States | Hematologic cancer | Retrospective cohort study | 143/823 | 65 years | NA | |
| Luetkens et al. [16] | 2020 | United States | MM | Case report | 1/0 | 72 years | F | 3 ASCT |
| Moore et al. [17] | 2020 | United States | NHL | Case report | 1/0 | 63 years | F | |
| Malsy et al. [18] | 2020 | Germany Croatia |
FL | Case report | 1/0 | 53 years | F | |
| Rnjak et al. [2] | 2021 | Russia | DLBCL | Case report | 1/0 | 53 years | M | ASCT |
| Balashov et al. [19] | 2021 | Russia | Juvenile myelomonocytic leukemia | Case report | 1/0 | 9 months | F | HSCT |
| Biernat et al. [20] | 2021 | Poland | Hematological malignancy (Acute leukemia/MDS, CLL, Aggressive Lymphoma, MM) | Retrospective cohort study | 23/22 | NA | 38% F, 62% M | |
| Çınar et al. [21] | 2020 | Turkey | MDS complicated by recently disseminated tuberculosis with associated kidney disease | Case report | 1/0 | 55 years | M | |
| Dell’Isola et al. [22] | 2021 | Italy | B-cell ALL | Case report | 1/0 | 6 years | F | |
| Ferrari et al. [23] | 2021 | Italy | FL | Case report | 7/0 | 48 years | F | |
| FL | 60 years | M | ||||||
| Indolent NHL | 60 years | M | SCT | |||||
| Primary myelofibrosis | 43 years | M | ||||||
| DLBL | 70 years | M | ||||||
| ALL | 69 years | M | SCT | |||||
| CLL | 60 years | M | ||||||
| Hueso et al. [24] | 2020 | France | B-cell lymphopenia | Observational multicenter study | 15/0 | 58 (35–77) | 5 F, 12 M | |
| Jeyaraman et al. [25] | 2021 | India | Hematological malignancies | Retrospective observational multicenter study | 33/0 | 62 years (18–80) | 10 F, 23 M | 1 ASCT |
| Karatas et al. [26] | 2020 | Turkey | Mixed cellularity classical Hodgkin lymphoma and peripheral T-cell lymphoma | Case report | 1/0 | 61 years | M | ASCT |
| Oliva et al. [27] | 2022 | Italy | non-Hodgkin’s lymphoma | Retrospective observational single center study | 6 | 59.5 years | 6F |
CPT: convalescent plasma treated, CLL: chronic lymphocytic leukemia, MML: myelomonocytic leukemia, MM: multiple myeloma, HIV: human immunodeficiency virus, DLBL: diffused large B-cell lymphoma, NHL: non-Hodgkin’s lymphoma, ALL: acute lymphocytic leukemia, FL: follicular lymphoma. SCT: stem cell transplant, ASCT: autologous stem cell transplant, HSCT: hematopoietic stem cell transplant, MDS: Myelodysplastic syndrome, M: male, F: female.
Figure 2.
The frequency of hematological malignancies reported in the identified articles. CLL: chronic lymphocytic leukemia, MML: myelomonocytic leukemia, MM: multiple myeloma, HIV: human immunodeficiency virus, DLBL: diffused large B-cell lymphoma, NHL: non-Hodgkin’s lymphoma, ALL: acute lymphocytic leukemia, FL: follicular lymphoma.
3.3. Dose, Time of Administration and Clinical Outcomes of CPT
The dose of CP varied from 200–300 mL per transfusion. CPT was administered at variable time-points after hospital admission, with some patients receiving CPT as early as day 2 post-diagnosis with COVID-19, while most of the remaining patients received CPT as a last treatment option after approximately one month of hospitalization (Table 3). In 14 studies, the total number of administered CPT doses ranged between 1–3 doses; however, two 53-year-old patients diagnosed with lymphoma were administered eight and 12 doses [2,18] due to their poor response to the initial treatment.
Table 3.
Summary of clinical outcomes from selected articles.
| Authors | Sample Sizes CPT/Non-CPT |
Day of CPT Administration | No. of CP Units (Dosage) | Outcome Endpoint | Outcome | Mortality | Adverse Events to CPT | Drugs |
|---|---|---|---|---|---|---|---|---|
| Shankar et al. [7] | 1/0 | Day 8 and 9 post illness onset | 2 (15 mL/kg) | 14 Days | Asymptomatic after 14 days | 0% | None | Oxygen therapy, Steroids (hydrocortisone and dexamethasone) |
| Szwebel et al. [13] | 1/0 | Day 65 and 66 post symptoms onset | 2 units daily | 70 Days | Asymptomatic after 70 days | 0% | None | Oxygen therapy, dexamethasone, oral prednisone, lopinavir/ritonavirm tocilizumab |
| Wright et al. [14] | 1/0 | NA | 1 (200 mL) | 1 month | Asymptomatic after 1 month and improvement of bilateral pulmonary infiltrates | 0% | None | Azithromycin and HCQ, oxygen therapy and supportive care |
| Tremblay et al. [8] | 24/0 | Median time: 3 days between doses | 2 (250 mL) | NA | 13 patients were discharged home, 1 patient still hospitalized, and 10 patients died | 41.7% | 3 patients had FNHTR | Oxygen therapy and HCQ or azithromycin or remdesiviror tocilizumab or combination |
| Thompson et al. [15] | 143/823 | NA | - | Significantly improved 30-day mortality | 13.3% vs. 24.8% | None | Corticosteroids, remdesivir, tocilizumab, and HCQ | |
| Luetkens et al. [16] | 1/0 | NA | 1 (200 mL) | 6 days | Asymptomatic at approximately 6 days from onset | 0% | None | Oxygen therapy |
| Moore et al. [17] | 1/0 | Day 88 post illness onset | 1 (200 mL) | 97 days | Asymptomatic at approximately 97 days from onset | 0% | None | Metoprolol for heart rate regulation and apixaban for anticoagulation |
| Malsy et al. [18] | 1/0 | Day 85 post illness onset | Two-course of 6 units (2 units/day administered every other day | 140 days | Asymptomatic at approximately 140 days from onset | 0% | None | Remdesivir |
| Rnjak et al. [2] | 1/0 | Day 48, 49, 54, 55, 56, 57, 105 and 109 post illness onset | 8 (~200 mL) | 129 days | Afebrile with regression of pneumonia at 129 days from onset | 0% | None | Oxygen therapy, remdesivir and steroids |
| Balashov et al. [19] | 1/0 | Day 146 post HSCT | 3 (10 mL/kg) | 4 months | Complete viral clearance, full resolution of the lung lesions on CT | 0% | None | Tocilizumab and methylprednisolone |
| Biernat et al. [20] | 23/22 | Day 2–3 after diagnosis | 1–2 (200–250 mL) | Day 14 | Milder infection, less severe and faster resolution of symptoms, viral clearance | 13.0% vs. 41.0% | None | Oxygen therapy, mechanical ventilation, HCQ, Dexamethasone, Remdesivir, Tocilizumab, Lopinavir/Ritonavir |
| Çınar et al. [21] | 1/0 | Day 5 post symptoms onset | 2 (200 mL) | Day 7 | Improved dyspnea and fever resolution, viral clearance | 0% | None | Tocilizumab and favipiravir |
| Dell’Isola et al. [22] | 1/0 | Day 10 post admission | 3 (10 mL/kg) | Day 18 | Viral clearance | 0% | None | Remdesivir and prednisone |
| Ferrari et al. [23] | 7/0 | NA | 3 (210 mL) | Day 2 | COVID-19 symptoms resolved, viral clearance, radiological improvement | 0% | None | Corticosteroid, HCQ, low-molecular-weight heparin, and antibiotics |
| NA | Day 2 | COVID-19 symptoms resolved, viral clearance, radiological improvement | Corticosteroid, HCQ, low-molecular-weight heparin, and antibiotics | |||||
| NA | Day 3 | COVID-19 symptoms resolved, radiological improvement | Corticosteroid, HCQ, low-molecular-weight heparin and antibiotics | |||||
| NA | Day 7 | COVID-19 symptoms resolved, viral clearance | Corticosteroid, HCQ, low-molecular-weight heparin, and antibiotics | |||||
| NA | N/A | COVID-19 symptoms resolved, viral clearance, radiological improvement | Corticosteroid, HCQ, low-molecular-weight heparin, and antibiotics | |||||
| NA | Day 7 | COVID-19 symptoms resolved, viral clearance, radiological improvement | Corticosteroid, HCQ, low-molecular-weight heparin, and antibiotics | |||||
| NA | Day 7 | COVID-19 symptoms resolved, viral clearance, radiological improvement | Corticosteroid, HCQ, low-molecular-weight heparin, and antibiotics | |||||
| Hueso et al. [24] | 15/0 | Day 0 + 1 (2 units each) | 4 (200–220 mL) | Day 2 | Fever resolved, and COVID-19 symptoms resolved after 2 weeks, decrease in RNAemia within 7–14 days | 5.9% | None | Remdesivir and tocilizumab |
| Jeyaraman et al. [25] | 33/0 | 4 days apart (range: 2–25 days) | 1–2 (200 mL) | Day 3 | Fever resolved | 45.5% | None | HCQ, remdesivir, favipiravir, other broad-spectrum antibiotics, steroids (methylprednisolone or dexamethasone), tocilizumab and oxygen support |
| Karatas et al. [26] | 1/0 | Day 40 post admission | 1 | Day 34 | Persistent SARS-CoV-2 viral shedding for 74 days | 0% | None | HCQ and azithromycin |
| Oliva et al. [27] | 6/0 | 51 post infection | 3 (300 mL) | 3–9 days | 5 survived and 1 death | 20% | 1 Transient sinustachycardia |
anti-CD20 drugs with different anti-viral medications for each patient |
CPT: convalescent plasma treated, HCQ: Hydroxychloroquine.
3.4. Assessment of Primary and Secondary Endpoints
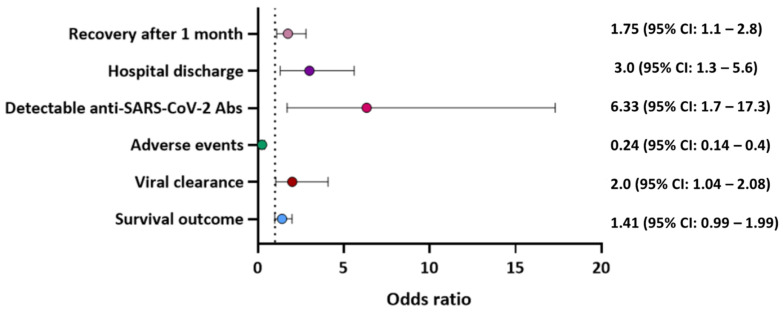
The primary goal of this study was measured by survival outcomes and mortality, development of adverse events, and hospital discharge. Survival rate was improved following CPT treatment. Indeed, mortality was reported in 56 (21.7%) patients who received CPT compared to 213 (25.2%) control patients who received the standard care. Furthermore, the use of CPT was associated with improved overall survival (OR: 1.41, 95%CI: 0.99–1.99), hospital discharge (OR: 3.0 95%CI: 1.3–5.6), and recovery after 1 month of CPT (OR: 1.75 95%CI: 1.1–2.8). Moreover, the probability of adverse events in these patients due to CPT was significantly lower compared to the control group (OR: 0.24 95%CI: 0.14–0.40) (Figure 3). Adverse reactions to CPT were reported in only three patients who developed a febrile non-haemolytic transfusion reaction. Other treatments that were administered to patients along with CPT included oxygen therapy, steroids, Azithromycin, Hydroxychloroquine, and Remdesivir.
Figure 3.
Odds ratio for the effectiveness of CPT on clinical outcome of patients with hematological malignancies and diagnosed with COVID-19.
The secondary goal was assessed by viral clearance. It was found that the use of CPT aids in viral clearance (OR: 2.0, 95%CI: 1.04–2.08). Furthermore, detectable anti-SARS-CoV-2 antibody titer (IgG and/or IgM) was quantified by either a chemiluminescent microparticle immunoassay method (cut-off 1.40 index S/C) or using ELISA kit (cut off > 1.1). There were more detectable anti-SARS-CoV-2 antibodies after CPT than in the control detection (OR: 6.33 95%CI: 1.7–17.3).
4. Discussion
In this study, we report on the clinical benefits of using CPT to treat COVID-19 infected patients with hematological malignancy. Our analysis of the currently published studies that included appropriate control groups suggests that CPT may be associated with improved overall survival (OR: 1.41, 95%CI: 0.99–1.99), viral clearance, (OR: 2.0, 95%CI: 1.04–2.08), detection of anti-SARS-CoV-2 antibodies in the patient’s plasma post-CPT (OR: 6.33 95%CI: 1.7–17.3), and recovery after 1 month of CPT (OR: 1.75 95%CI: 1.1–2.8). Furthermore, the probability of adverse events due to CPT was low (OR: 0.24 95%CI: 0.14–0.40). Further studies with appropriate control groups are warranted to fully elucidate the effect of CPT on outcomes in COVID-19 patients with hematological malignancies.
The majority of the studies in the literature reported on the effect of CPT on COVID-19 outcomes in adult patients with hematological malignancies, and there are very limited published case reports in pediatric patients. Children with malignancies have always been at high risk of infections due to anti-cancer treatments resulting in an underlying immunocompromised state. Although several studies have reported less severe COVID-19 disease in children than adult cancer patients, there have been some reports of severe disease in children with cancer [7]. A few case reports have demonstrated improved outcomes following CPT in children with hematology malignancies [7,19,22].
A high mortality rate has been reported in hematological malignancy patients with severe COVID-19 [28]. The efficacy of CPT to reduce the mortality rate was reported in four of the 17 studies; however, only two studies compared the percentage of mortality to the control group. Thompson et al. reported a mortality rate of 13.1% in the CPT recipient group and 24.8% in the non-recipient group. Moreover, the mortality rate was significantly lower in intensive care unit patients on mechanical ventilation [15]. Furthermore, another study demonstrated a mortality rate of 13% among the CPT recipient group compared to a mortality rate of 41% in the non-recipient group [20].
A recent meta-analysis reported that using CPT could significantly reduce the risk of mortality in COVID-19 patients compared to those without CPT [29]. Jeyaraman et al. [25] and Tremblay et al. [8] reported a mortality rate of 45.5% and 35.7%, respectively. However, the efficacy of CPT to reduce mortality in these two studies is difficult to ascertain due to the lack of a control group. Abeldaño Zuñiga et al. [30] concluded that using CPT for hospitalized COVID-19 patients could result in clinical improvement for patients; however, it does not significantly lower mortality rates in comparison to standard care and placebo.
Most of the articles included in our analysis reported significant improvement in patient status after CPT, and patients become asymptomatic and were discharged. Four articles reported improved viral clearance after using CPT [19,22].
In all the reviewed articles, we found that CPT is safe, and no adverse reactions were reported in patients with hematological malignancies presenting with severe COVID-19 infection. However, one of the studies reported a febrile non-hemolytic transfusion reaction (FNHTR) in three patients post-CPT [8]. Supporting our findings, a recent meta-analysis concluded that CPT is a safe approach for COVID-19 patient therapy, with only 3.5% of recipients patients experiencing an adverse reaction [31]. Nevertheless, a systematic review stated that it is difficult to determine whether the adverse effect is due to the patient’s condition or CPT [32]. Therefore, more studies are required to investigate the safety of CPT in COVID-19 infected cancer patients.
A minimum of one ABO compatible CP dose was given to patients, with most receiving one to two doses. However, one patient with diffuse large B-cell lymphoma (DLBCL) who had a prolonged active COVID-19 infection for 129 days received eight doses of CP until remission [2]. There are no recommended or standardized doses of CP; however, most of the studies used one to two units, and the titer of the antibody might determine the optimal dose [33]. In our study, only four papers measured the titer of the donor antibodies before transfusion [8,19,22,26]. One of the included retrospective multicenter observational studies found that there were no significant differences in mortality in patients who received one versus two doses of CP or in patients who received early versus late transfusion of CP [25].
In all 18 studies, standard care including supportive care and antiviral therapy with hydroxychloroquine, azithromycin, and/or ritonavir, and Tocilizumab (for very critical patients) were given to patients before initiating CPT. In addition, most of the patients required oxygen therapy. Duan et al. reported that CPT combined with supportive care and antiviral therapy can improve the clinical outcome of COVID-19 patients [34]. Additionally, Agarwal et al. reported that using CPT alone might not be effective in reducing the severity, risk of mortality, and period of hospitalization of COVID-19 patients [35]. Therefore, it is recommended that CPT therapy be used as part of a combination of treatment to achieve the best result. To the best of our knowledge, this is one of the first systematic reviews to discuss the efficacy and safety of CPT in patients with hematological malignancies presenting with severe COVID-19 complications.
5. Limitations
Our meta-analysis has several limitations. First, the CPT treatment protocol had not been widely reviewed, and currently no clinical guidelines on the use of CPT, including the dose and donor antibody titer, are established for COVID-19 patients. Therefore, this has led to different CPT regimes. Furthermore, due to the lack of established clinical guidelines, CPT treatment outcomes are evaluated in multiple parameters.
Second, we were unable to perform the I2 statistic for heterogeneity because the number of studies included is small. Considerable heterogeneity may exist in this study due to the lack of an appropriate control group in most of the included studies and randomized control trials. Furthermore, the heterogeneity of CPT dose, time of transfusion, reported outcome such as viral clearance, donor antibody titers, and lack of control groups to compare the result made it difficult to perform a meta-analysis.
6. Conclusions
This review suggests that CPT may be used as a safe supportive therapy for patients with hematological malignancies and diagnosed with COVID-19 infection. The exact mechanism by which CPT may have mediated improved outcomes in the treated patients is likely multifactorial and could include reduction in viral load via enhanced clearance. Further studies and analysis are needed to fully understand the effectiveness of CPT in cancer patients with COVID-19.
Author Contributions
Conceptualization, S.S.; methodology, I.A. and H.A.-J.; formal analysis, S.S., I.A. and H.A.-J.; resources, A.M.A.; writing—original draft preparation, I.A. and H.A.-J.; writing—review and editing, A.M.A. and S.S.; supervision, S.S. and A.M.A.; funding acquisition, A.M.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there are no conflict of interest.
Funding Statement
This study was supported by Qatar University internal grant No (QUST-1-CHS-2022-322). The findings achieved herein are solely the responsibility of the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., et al. Author Correction: A New Coronavirus Associated with Human Respiratory Disease in China. Nature. 2020;580:E7. doi: 10.1038/s41586-020-2202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rnjak D., Ravlić S., Šola A.M., Halassy B., Šemnički J., Šuperba M., Hećimović A., Kurolt I.C., Kurtović T., Mačak Šafranko Ž. COVID-19 convalescent plasma as long-term therapy in immunodeficient patients? Transfus. Clin. Biol. 2021;28:264–270. doi: 10.1016/j.tracli.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decker A., Welzel M., Laubner K., Grundmann S., Kochs G., Panning M., Thimme R., Bode C., Wagner D., Lother A. Prolonged SARS-CoV-2 Shedding and Mild Course of COVID-19 in a Patient after Recent Heart Transplantation. Am. J. Transpl. 2020;20:3239–3245. doi: 10.1111/ajt.16133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemal R., Tournilhac O., Bay J.O. Biological Markers in Haematological Malignancies. Bull Cancer. 2014;101:7–11. doi: 10.1684/bdc.2014.1974. [DOI] [PubMed] [Google Scholar]
- 6.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., Shete S., Hsu C.-Y., Desai A., Lima Lopes G., Jr. Clinical Impact of COVID-19 on Patients with Cancer (CCC19): A Cohort Study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar R., Radhakrishnan N., Dua S., Arora S., Rana M., Sahu D.K., Rai S., Gupta D.K. Convalescent Plasma to Aid in Recovery of COVID-19 Pneumonia in a Child with Acute Lymphoblastic Leukemia. Transfus. Apher. Sci. 2021;60:102956. doi: 10.1016/j.transci.2020.102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tremblay D., Seah C., Schneider T., Bhalla S., Feld J., Naymagon L., Wang B., Patel V., Jun T., Jandl T. Convalescent Plasma for the Treatment of Severe COVID-19 Infection in Cancer Patients. Cancer Med. 2020;9:8571–8578. doi: 10.1002/cam4.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharadwaj K.K., Sarkar T., Ghosh A., Baishya D., Rabha B., Panda M.K., Nelson B.R., John A.B., Sheikh H.I., Dash B.P. Macrolactin A as a Novel Inhibitory Agent for SARS-CoV-2 M(Pro. Bioinformatics Approach. Appl. Biochem. Biotechnol. 2021;193:3371–3394. doi: 10.1007/s12010-021-03608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizk J.G., Forthal D.N., Kalantar-Zadeh K., Mehra M.R., Lavie C.J., Rizk Y., Pfeiffer J.P., Lewin J.C. Expanded Access Programs, Compassionate Drug Use, and Emergency Use Authorizations during the COVID-19 Pandemic. Drug Discov. Today. 2021;26:593–603. doi: 10.1016/j.drudis.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Bull. World Health Organ. 2007;85:867–872. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaushik A., Gupta S., Sood M., Sharma S., Verma S. A Systematic Review of Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 Infection. Pediatr. Infect. Dis. J. 2020;39:340–346. doi: 10.1097/INF.0000000000002888. [DOI] [PubMed] [Google Scholar]
- 13.Szwebel T.A., Veyer D., Robillard N., Eshagh D., Canoui E., Bruneau T., Contejean A., Azoulay C., Serrano T., Hueso T. Usefulness of Plasma SARS-CoV-2 RNA Quantification by Droplet-Based Digital PCR to Monitor Treatment Against COVID-19 in a B-Cell Lymphoma Patient. Stem. Cell Rev. Rep. 2021;17:296–299. doi: 10.1007/s12015-020-10107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright Z., Bersabe A., Eden R., Bradley J., Cap A. Successful Use of COVID-19 Convalescent Plasma in a Patient Recently Treated for Follicular Lymphoma. Volume 21. Elsevier Inc.; Philadelphia, PA, USA: 2021. Clin. Lymphoma Myeloma Leuk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson M.A., Henderson J.P., Shah P.K., Rubinstein S.M., Joyner M.J., Choueiri T.K., Flora D.B., Griffiths E.A., Gulati A.P., Hwang C. Association of Convalescent Plasma Therapy with Survival in Patients with Hematologic Cancers and COVID-19. JAMA Oncol. 2021;7:1167–1175. doi: 10.1001/jamaoncol.2021.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luetkens T., Metcalf R., Planelles V., Zheng Y., Larragoite E.T., Spivak E.S., Spivak A.M., Steinbach M., Blaylock R.C., Avila S.V. Successful transfer of anti-SARS-CoV-2 immunity using convalescent plasma in an MM patient with hypogammaglobulinemia and COVID-19. Blood Adv. 2020;4:4864–4868. doi: 10.1182/bloodadvances.2020002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore J.L., Ganapathiraju P.V., Kurtz C.P., Wainscoat B. A 63-Year-Old Woman with a History of Non-Hodgkin Lymphoma with Persistent SARS-CoV-2 Infection Who Was Seronegative and Treated with Convalescent Plasma. Am. J. Case Rep. 2020;21:927812. doi: 10.12659/AJCR.927812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malsy J., Veletzky L., Heide J., Hennigs A., Gil-Ibanez I., Stein A., Lütgehetmann M., Rosien U., Jasper D., Peine S. Sustained Response after Remdesivir and Convalescent Plasma Therapy in a B-Cell Depleted Patient with Protracted COVID-19. Clin. Infect. Dis. 2020;73:4020–4024. doi: 10.1093/cid/ciaa1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balashov D., Trakhtman P., Livshits A., Kovalenko I., Tereshenko G., Solopova G., Petraikina E., Maschan A., Novichkova G. SARS-CoV-2 convalescent plasma therapy in pediatric patient after hematopoietic stem cell transplantation. Transfus. Apher. Sci. 2021;60:102983. doi: 10.1016/j.transci.2020.102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biernat M.M., Kolasińska A., Kwiatkowski J., Urbaniak-Kujda D., Biernat P., Janocha-Litwin J., Szymczyk-Nużka M., Bursy D., Kalicińska E., Simon K. Early Administration of Convalescent Plasma Improves Survival in Patients with Hematological Malignancies and COVID-19. Viruses. 2021;13:436. doi: 10.3390/v13030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Çınar O.E., Sayınalp B., Aladağ Karakulak E., Avşar Karataş A., Velet M., İnkaya A.Ç., Ersoy Ortaç N.E., Öcal S., Aksu S., Haznedaroğlu İ.C. Convalescent (immune) plasma treatment in a myelodysplastic COVID-19 patient with disseminated tuberculosis. Transfus. Apher. Sci. 2020;59:102821. doi: 10.1016/j.transci.2020.102821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dell’Isola G.B., Felicioni M., Ferraro L., Capolsini I., Cerri C., Gurdo G., Mastrodicasa E., Massei M.S., Perruccio K., Brogna M. Case Report: Remdesivir and Convalescent Plasma in a Newly Acute B Lymphoblastic Leukemia Diagnosis With Concomitant Sars-CoV-2 Infection. Front. Pediatr. 2021;9:712603. doi: 10.3389/fped.2021.712603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrari S., Caprioli C., Weber A., Rambaldi A., Lussana F. Convalescent Hyperimmune Plasma for Chemo-Immunotherapy Induced Immunodeficiency in COVID-19 Patients with Hematological Malignancies. Leuk Lymphoma. 2021;62:1490–1496. doi: 10.1080/10428194.2021.1872070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hueso T., Pouderoux C., Péré H., Beaumont A.-L., Raillon L.-A., Ader F., Chatenoud L., Eshagh D., Szwebel T.-A., Martinot M. Convalescent Plasma Therapy for B-Cell-Depleted Patients with Protracted COVID-19. Blood. 2020;136:2290–2295. doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeyaraman P., Agrawal N., Bhargava R., Bansal D., Ahmed R., Bhurani D., Bansal S., Rastogi N., Borah P., Naithani R. Convalescent Plasma Therapy for Severe Covid-19 in Patients with Hematological Malignancies. Transfus. Apher. Sci. 2021;60:103075. doi: 10.1016/j.transci.2021.103075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karataş A., İnkaya A.Ç., Demiroğlu H., Aksu S., Haziyev T., Çınar O.E., Alp A., Uzun Ö., Sayınalp N., Göker H. Prolonged Viral Shedding in a Lymphoma Patient with COVID-19 Infection Receiving Convalescent Plasma. Transfus. Apher. Sci. 2020;59:102871. doi: 10.1016/j.transci.2020.102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliva A., Cancelli F., Brogi A., Curtolo A., Savelloni G., Siccardi G., Marcelli G., Mazzuti L., Ricci P., Turriziani O. Convalescent Plasma for Haematological Patients with SARS-CoV-2 Pneumonia and Severe Depletion of B-Cell Lymphocytes Following Anti-CD20 Therapy: A Single-Centre Experience and Review of the Literature. New Microbiol. 2022;45:62–72. [PubMed] [Google Scholar]
- 28.Borah P., Mirgh S., Sharma S.K., Bansal S., Dixit A., Dolai T.K., Lunkad S., Gupta N., Singh G., Jain A. Effect of Age, Comorbidity and Remission Status on Outcome of COVID-19 in Patients with Hematological Malignancies. Blood Cells Mol. Dis. 2021;87:102525. doi: 10.1016/j.bcmd.2020.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wardhani S.O., Fajar J.K., Wulandari L., Soegiarto G., Purnamasari Y., Asmiragani A., Maliga H.A., Ilmawan M., Seran G., Iskandar D.S. Association between Convalescent Plasma and the Risk of Mortality among Patients with COVID-19: A Meta-Analysis. F1000Research. 2021;10:64. doi: 10.12688/f1000research.36396.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abeldaño Zuñiga R.A., Coca S.M., Abeldaño G.F., González-Villoria R.A.M. Clinical Effectiveness of Drugs in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Ther. Adv. Respir. Dis. 2021;15:17534666211007214. doi: 10.1177/17534666211007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barreira D.F., Lourenço R.A., Calisto R., Moreira-Gonçalves D., Santos L.L., Videira P.A. Assessment of the Safety and Therapeutic Benefits of Convalescent Plasma in COVID-19 Treatment: A Systematic Review and Meta-Analysis. Front. Med. 2021;8:378. doi: 10.3389/fmed.2021.660688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valk S.J., Piechotta V., Kimber C., Chai K.L., Monsef I., Doree C., Wood E.M., Lamikanra A.A., Roberts D.J., McQuilten Z. Convalescent Plasma and Hyperimmune Immunoglobulin to Prevent Infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2021;10:CD01380. doi: 10.1002/14651858.CD013600.pub3. [DOI] [PubMed] [Google Scholar]
- 33.Bloch E.M. In: COVID-19: Convalescent Plasma and Hyperimmune Globulin. Kleinman S., Tirnauer J.S., Feldweg T.M., editors. UpToDate; Waltham, MA, USA: 2021. [Google Scholar]
- 34.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y. Effectiveness of Convalescent Plasma Therapy in Severe COVID-19 Patients. Proc. Natl. Acad. Sci. USA. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P. Convalescent plasma in the management of moderate COVID-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.