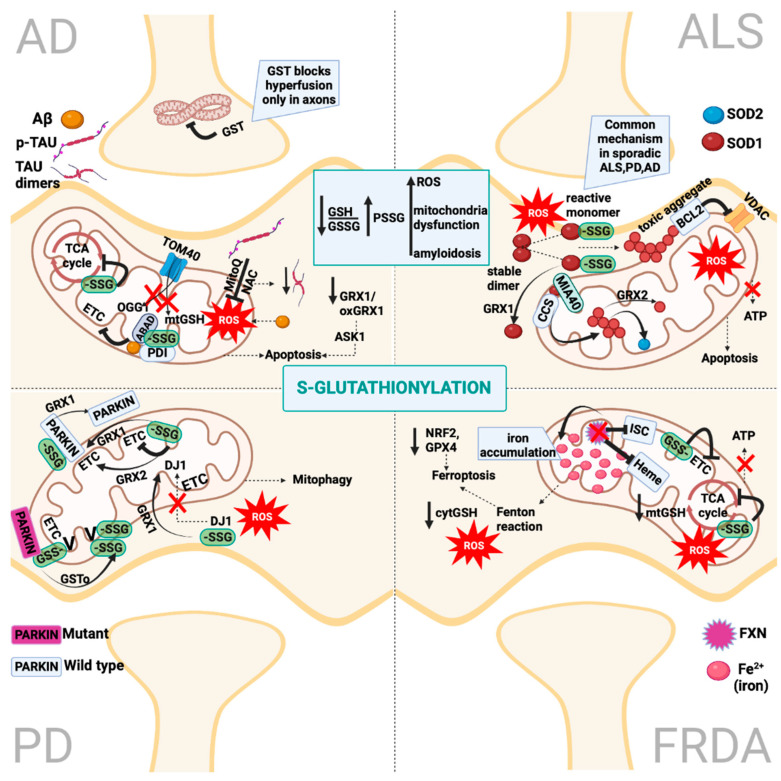
Figure 6.
S-Glutathionylation impairs mitochondria homeostasis in AD, PD, ALS and FRDA. In AD, aberrant S-glutathionylation due to decreased levels of cytosolic and mitochondrial GSH inhibits the TCA cycle and ETC, impairing mitochondria respiration. Polymorphisms in the TOMM40 gene inhibit the import of GSH and OGG1 inside mitochondria. SOD1 has been found inside the mitochondria matrix and is colocalized with S-glutathionylated ABAD and PDI, interfering with ETC functions. The addition of MitoQ/NAC decreases the ROS production associated with phosphorylated Tau in AD models but decreases the amount of dimeric Tau. Both reduced levels of GRX1 and oxidized GRX1 cause ASK1-mediated apoptosis in AD. In PD, the S-glutathionylation of ETC inhibits its activity, which is reversed by GRX1 and GRX2. The S-glutathionylation of DJ1 inhibits its translocation to mitochondria, while GRX1 reverses this process. The S-glutathionylation of PARKIN can be reversed by GRX1, while the mutated PARKIN-mediated mitophagy decreased the function of ATP synthase (Complex V) and this can be reversed by GSTo, which increases its glutathionylation in normal levels. In ALS, the S-glutathionylation of SOD1 triggers stable dimer dissociation and misfolding reactive monomers. This process can be reversed by GRX1. SOD1’s toxic aggregates interact with BCL2, and this leads to VDAC inhibition and the ablation of ATP production. SOD1 and CCS can be trapped in the mitochondria’s matrix by disulfide dimerization induced by MIA40, leading to the aggregation of SOD1 inside mitochondria, which, in turn, activates SOD2. GRX2 can reverse SOD1 oligomerization. In FRDA, frataxin deficiency leads to iron accumulation inside the mitochondria and inhibits heme synthesis and iron–sulfur cluster (ISC) biogenesis. The S-glutathionylation of ETC and TCA-cycle enzymes inhibits mitochondria respiration. Increased levels of ROS, iron accumulation, decreased levels of reduced glutathione and the downregulation of NRF2 and GPX4 trigger ferroptosis as the main cell death mechanism in FRDA. Created with BioRender.com.