Figure 1.
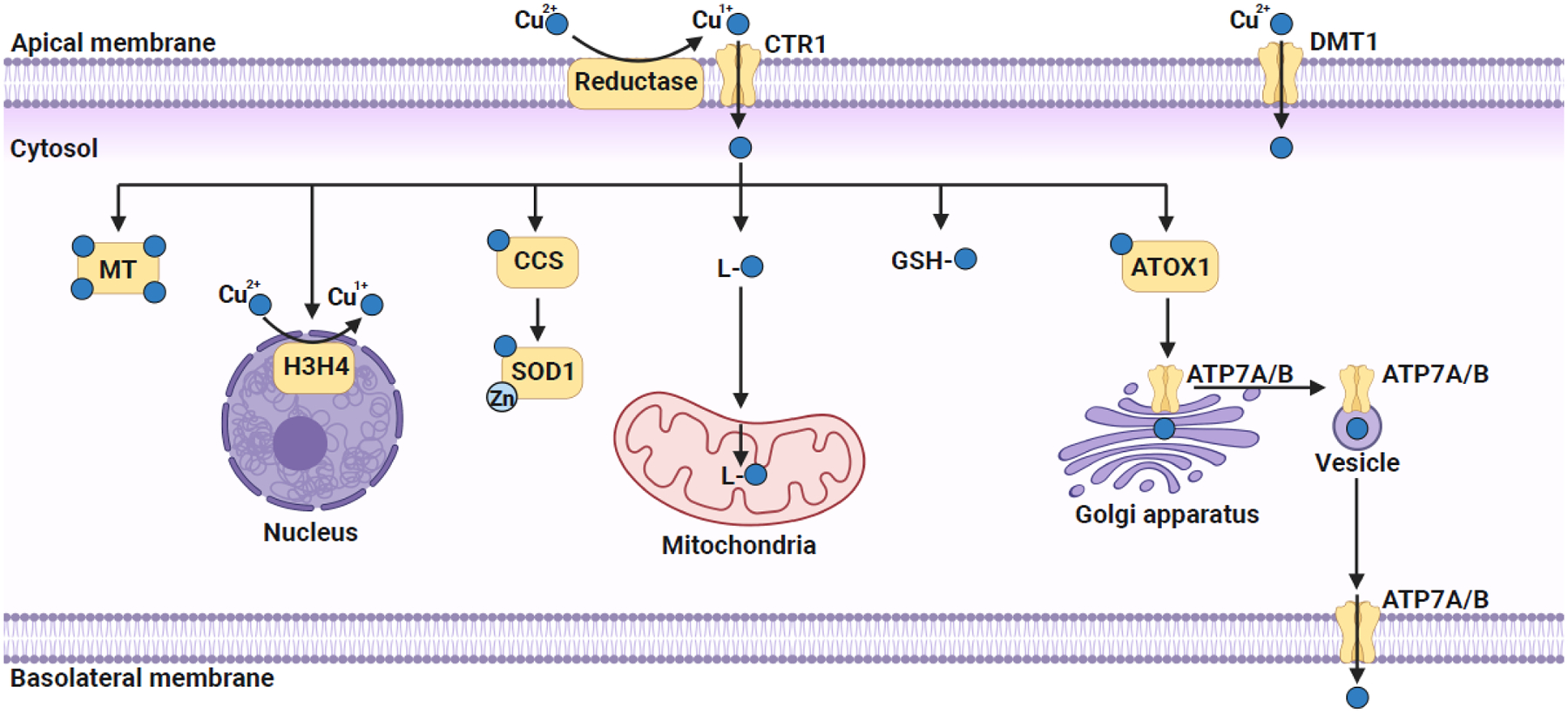
Copper trafficking in a polarized mammalian cell.
Extracellular Cu2+ is reduced to Cu1+ by plasma membrane-localized reductases that allows its import via the high-affinity plasma membrane copper transporter CTR1. Extracellular Cu2+ could also be imported by a non-specific divalent metal transporter DMT1. Once in the cytosol, copper is immediately bound to either a non-proteinaceous ligand (GSH or CuL) or a metallochaperone for subsequent trafficking to various organelles. CuL is proposed to transport copper to mitochondria, where it is stored within the mitochondrial matrix and acts as a feedstock for the metallation of mitochondrial cuproenzymes. In the cytosol, CCS, a metallochaperone, binds and transfers copper to Cu/Zn SOD1. Copper is delivered to the Golgi lumen, via the combined action of cytosolic metallochaperone ATOX1 and the Golgi membrane localized copper pumps ATP7A and ATP7B. Recently, histones H3–H4 tetramer have been shown to act as copper reductases that increase the bioavailable copper pool by catalyzing the reduction of Cu2+ to Cu1+. Excess copper is either bound to metallothionein (MT) or pumped out of the cell via the action of ATP7A/B, which translocates to the plasma membrane via vesicular trafficking. The figure was created with Biorender.com.
