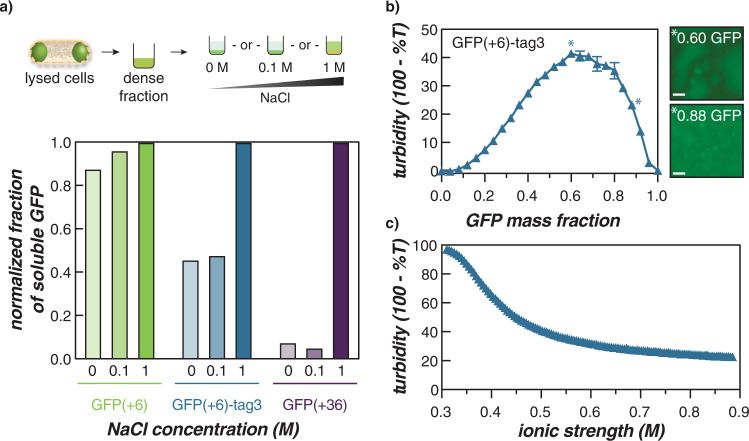
Fig. 4. In vitro characterization of GFP(+6)-tag3/RNA coacervates.
a The schematic (top) summarizes the solubility of GFP(+6)-tag3 intracellular condensates in buffers of increasing ionic strength. Similar to the highly supercharged, condensate-forming variant—GFP(+36)—GFP(+6)-tag3 was extracted from condensates ex vivo by increasing NaCl concentration, while a variant, GFP(+6) that did not form condensates at this time did not show preferential extraction as a function of salt concentration (bottom). Scheme adapted with permission from ACS Cent. Sci. 2020, 6(12), 2301–2310. Copyright 2020 American Chemical Society. b Turbidimetry measurements of GFP(+6)-tag3 with total RNA from torula yeast at 1 mg/ml total macromolecule concentration. Data points represent the average and error bars represent the standard deviation of three technical replicates. Microscopy images (right) depict the GFP channel image of the GFP/RNA mixture at 0.6 (top) or 0.88 (bottom) GFP mass fraction, which demonstrated the highest turbidity or formation of individual droplets that did not coalesce on the surface of the plate, respectively. Scale bars are 10 µm. c NaCl titration was conducted at 0.6 GFP mass fraction in 1 ml total volume of GFP/RNA mixture prepared from 1 mg/ml protein and RNA stocks. Both turbidity and salt stability assays were conducted in a physiological buffer that mimics the intracellular ion concentration in E. coli (70 mM K2HPO4, 60 mM KCl, 40 mM NaCl, pH 7.4).