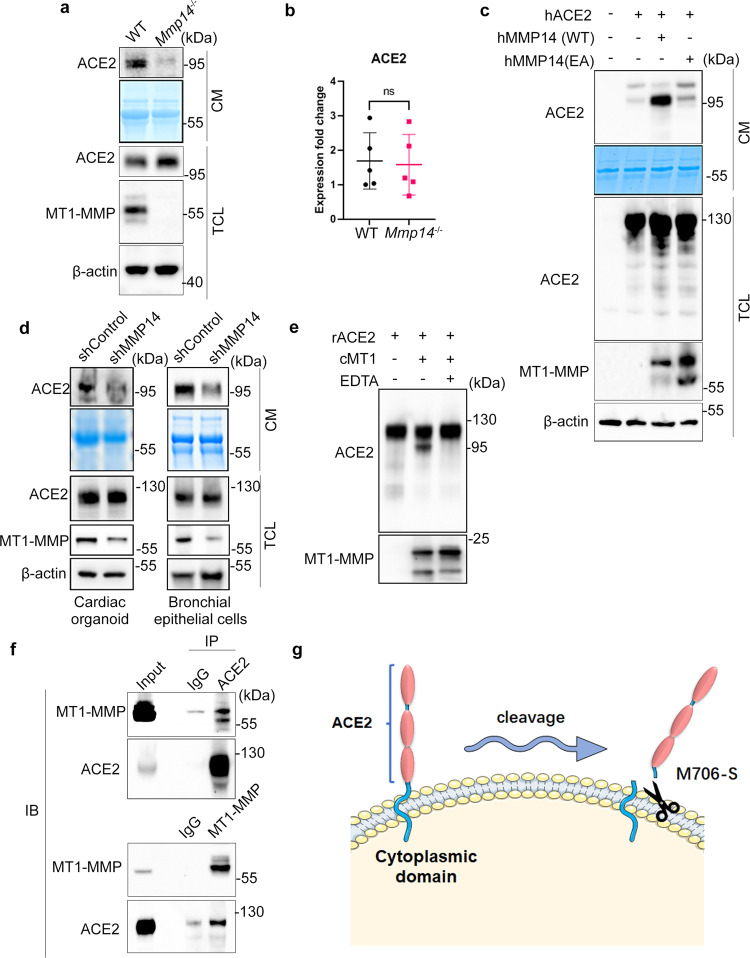
Fig. 1. The shedding of ACE2 by MT1-MMP.
a Western blotting analyses on the expression of ACE2 in the total cell lysate (TCL) and conditioned medium (CM) derived from wild-type and Mmp14-/- mouse primary lung epithelial cells. Coomassie stained membrane served as a loading control for conditioned medium (n = 3). b Real-time quantitative PCR analyses on the relative mRNA expression of ACE2 in wild-type and Mmp14-/- mouse primary lung epithelial cells (n = 5). c HEK293T cells were co-transfected with human ACE2 and wild-type or E/A catalytic mutant MT1-MMP (MT1 E240A). Expression of full-length ACE2 in total cell lysates and conditioned medium was detected by western blotting. Coomassie stained membrane was a loading control for conditioned medium (n = 3). d Western blotting analyses on the expression of solACE2 and memACE2 in human cardiac organoids and human bronchial epithelial cells that were lentivirally transduced with either shRNA or shMMP14 (n = 3). e The recombinant full-length ACE2 (rACE2) was incubated with the catalytic domain of MT1-MMP (cMT1) and the protein mixture was analyzed by western blotting using specific antibodies (n = 3). f Endogenous interaction between MT1-MMP and ACE2. Co-immunoprecipitation was performed with the cell lysates of Caco2 cells and examined by western blotting analyses using specific antibodies. IgG immunoprecipitates were used as controls (n = 3). g A diagram illustrating the cleavage of ACE2 by MT1-MMP. Data are means ± S.E.M. of three independent repeats; two-tailed unpaired t-test for (b). Source data are provided as a Source Data file.