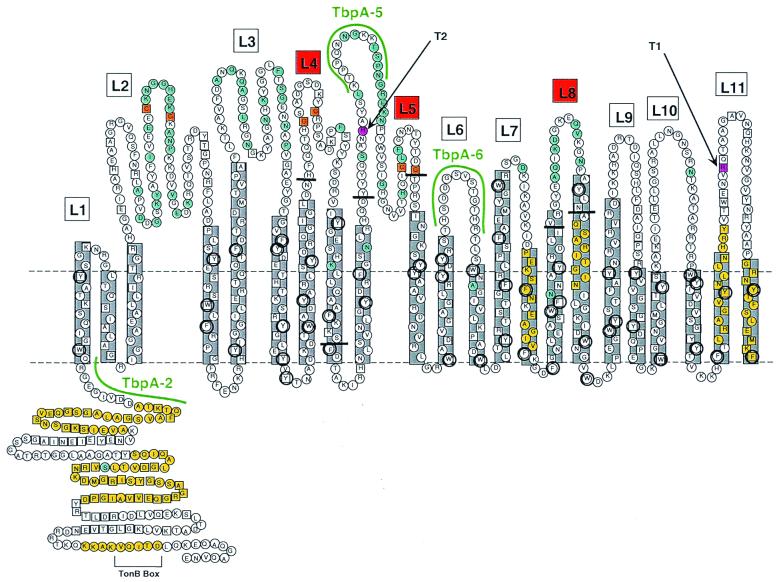
FIG. 1.
Putative topology model of gonococcal TbpA. Horizontal dotted lines represent the planes of the gonococcal outer membrane. The predicted amphipathic β-strands (22 in total) are shown in gray boxes. Putative surface exposed loops are numbered L1 to L11. Loops 4, 5, and 8 are indicated by red boxes, and the endpoints of the ΔL4, ΔL5, and ΔL8 deletions are indicated by short horizontal black bars. Residues that are conserved among all 17 TbpAs sequenced to date (12) are indicated by the squares. Residues that fall within previously characterized TonB homology domains (16) are shaded yellow. Residues that are variant among a panel of 5 gonococcal TbpAs (12) are shaded blue. Aromatic residues are circled. Cysteine residues are shaded orange. Two predicted trypsin cleavage sites are shaded pink. Although the TbpA sequence contains many other predicted trypsin cleavage sites, cleavage at these two sites would generate proteolytic cleavage products that most closely match the observed sizes of trypsin digest products T1 and T2 (see text). The sequences of three peptides used to generate antipeptide antibodies (12) are highlighted in green.