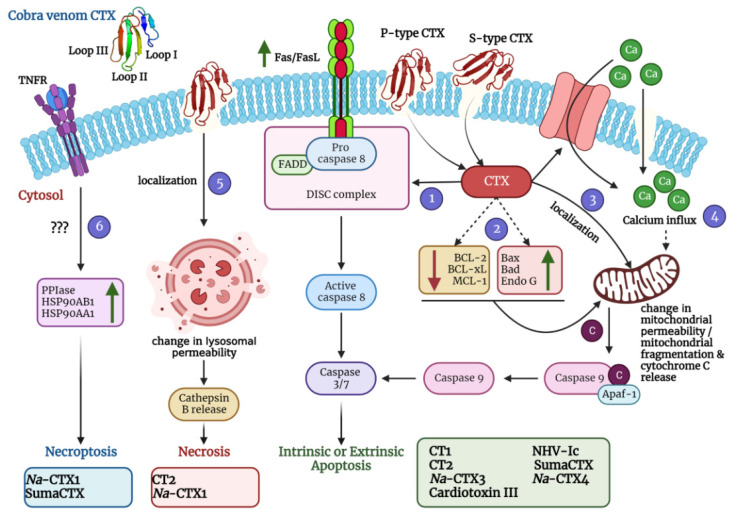
Figure 4.
A schematic diagram depicting diverse mechanisms of action of cobra venom CTXs was created using BioRender.com. Molecular dynamics simulations with representative members of S-type CTX have indicated that this class of CTXs incorporate loop I or both loops I and II into the lipid bilayer membrane for cellular entry. On the contrary, P-type CTXs interact with the lipid membranes via all three loops (I-III). After pore formation and entry into the cells, CTXs can adopt various mechanisms that eventually lead to apoptosis, necrosis, and necroptosis of the affected cells. They can induce the upregulation of FasL/Fas expression (via activation of the Ca2+/NOX4/ROS/p38 MAPK signaling axis) thereby activating the extrinsic pathway of apoptosis (Pathway 1). They can also induce the upregulation of apoptotic proteins and a concomitant downregulation of anti-apoptotic proteins to stimulate intrinsic apoptosis (Pathway 2). Moreover, they can localize in the mitochondria and change the mitochondrial membrane permeability leading to release of cytochrome c and subsequent intrinsic apoptosis activation (Pathway 3). CTXs can also increase the influx of calcium ions either via calcium channels or through non-selective pores to perturb the mitochondrial membrane permeability thereby leading to intrinsic apoptosis (Pathway 4). They can also localize in the lysosomes and increase the release of cathepsin B for inducing necrosis (Pathway 5). In addition, CTXs can also activate the necroptotic cell death pathway via upregulation of peptidyl–prolyl isomerase (PPIase) and heat shock proteins (HSP90AB1 and HSP90AA1) (Pathway 6). For exact mechanism of individual CTX, please refer to Table 2. Na-CTX1, Na-CTX3, and Na-CTX4 represent CTX isoforms isolated from the venom of N. atra.