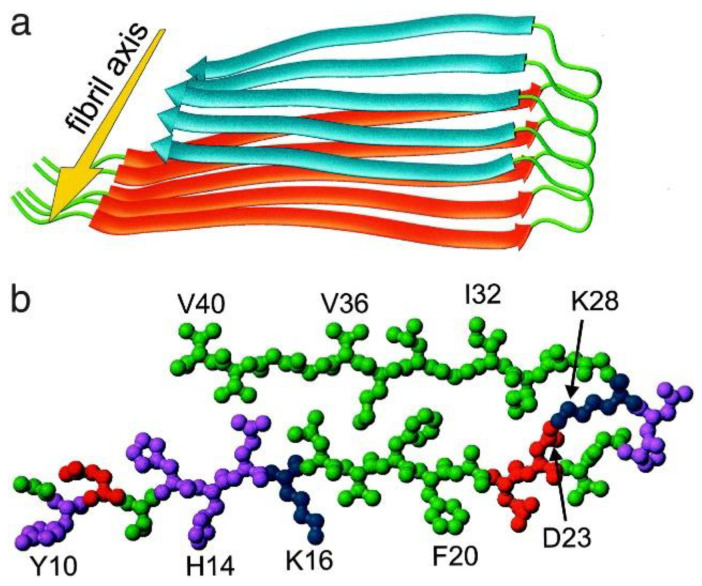
Figure 1.
Structural model for Aβ40 fibrils, consistent with solid-state NMR constraints on the molecular conformation and intermolecular distances and incorporating the cross-β motif common to all amyloid fibrils [17]. Residues 1–8 are considered fully disordered and are omitted. (a) Schematic representation of a single molecular layer or cross-β unit. The yellow arrow indicates the direction of the long axis of the fibril, which coincides with the direction of intermolecular backbone hydrogen bonds. The cross-β unit is a double-layered structure, with in-register parallel β-sheets formed by residues 12–24 (orange ribbons) and 30–40 (blue ribbons). Mass-per-length measurements from electron microscopy indicate that fibrils are formed by two parallel cross-β units. (b) Central Aβ40 molecule viewed down the long axis of the fibril. Residues are color-coded according to their sidechains as hydrophobic (green), polar (magenta), positively charged (blue), or negatively charged red).