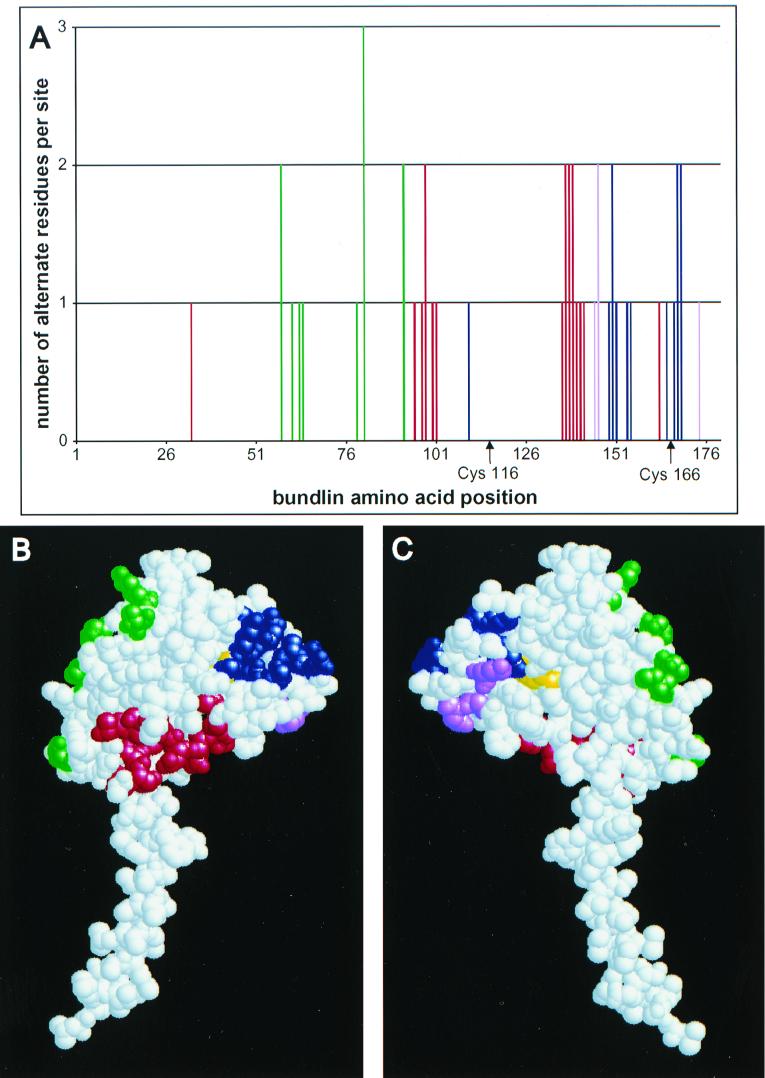
FIG. 6.
Variable amino acids may be clustered on the surface of bundlin. (A) Graphical analysis of bundlin variation. The bars represent amino acid residues that vary among the eight bundlin types identified in this study. The heights of the bars are proportional to the numbers of alternate residues per site. The positions of conserved cysteine residues are indicated below the x axis. (B and C) Theoretical 3D structure of bundlin (Chattopadhyaya and Ghose, unpublished). Colored amino acid residues are those that vary between bundlin types. These residues have been assigned to four color groups (green, red, blue, and violet) in accordance with their relative locations in the molecule. These colors correspond to those displayed in the graph (A). The disulfide-bonded cysteines are yellow. Panel B shows the convex face of bundlin that is likely to be facing outward on the surface of the BPF filament. Panel C shows the flattened face on the opposite side of the molecule that is likely to be facing toward the center of the filament, based on existing models of gonococcal and V. cholerae pili.