Abstract
(1) Introduction: There have been numerous reports on the neuroinvasive competence of SARS-CoV-2. Here, we present a case with anti-MOG positive bilateral optic neuritis and brainstem encephalitis secondary to COVID-19 infection. Additionally, we present a review of the current literature regarding the manifestation of anti-MOG positive optic neuritis as well as anti-MOG positive encephalitis after COVID-19 infection. (2) Case Report: A 59-year-old female patient, with a recent history of COVID-19 infection, presented a progressive reduction of visual acuity and bilateral retrobulbar pain for the last 20 days. An ophthalmological examination revealed a decreased visual acuity (counting fingers) and a bilateral papilledema. An MRI scan of the brain revealed a mild thickening of the bilateral optic nerves and high-intensity lesions in the medial and right lateral pons. A high titer of IgG and IgM antibodies against SARS-CoV-2 in serum and antibodies against myelin oligodendrocyte glycoprotein (anti-MOG) in serum and CSF were revealed. The diagnosis of anti-MOG brainstem encephalitis and optic neuritis was set. (3) Conclusions: The history of COVID-19 infection should raise awareness about these autoimmune and infection-triggered diseases, such as anti-MOG antibody disease.
Keywords: COVID-19, case report, Anti-MOG, optic neuritis, brainstem encephalitis
1. Introduction
Despite the fact that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mainly affects the respiratory system and produces corresponding respiratory tract symptoms, since the outbreak of the pandemic in December 2019, there have been numerous reports of the neuroinvasive potential of SARS-CoV-2. The disease mechanisms reported so far include the direct infection, para- and post-infectious, as well as vascular, mechanisms [1]. Manifestations such as encephalitis, acute demyelinating encephalomyelitis (ADEM), myelitis, immune-mediated central nervous system (CNS) and peripheral nervous system (PNS) demyelination, and cerebrovascular disease have been reported to date [2,3,4]. However, several other neurological symptoms during the course of the disease have also been reported. Concerning optic neuritis, several cases have been published; however, only six patients were positive for antibodies against myelin oligodendrocyte glycoprotein (anti-MOG). Of these, one patient had a presumed COVID-19 infection, while the rest had a confirmed one [5,6,7,8,9,10]. Moreover, concerning encephalitis, only a few cases with the presence of anti-MOG in serum after COVID-19 infection have been reported [3,4].
Here, we report a case of a female patient with bilateral optic neuritis and brainstem encephalitis secondary to COVID-19 infection. Additionally, we present a review of the current literature regarding the manifestation of anti-MOG positive optic neuritis as well as anti-MOG positive encephalitis after COVID-19 infection.
2. Case Presentation
A 59-year-old female patient with a history of hypertension and anxiety disorder, and an unremarkable family history, was referred to the Neurological Emergency Department of the University Hospital of Larissa, a tertiary hospital of central Greece, due to a progressive reduction of visual acuity and bilateral retrobulbar pain for the last 20 days. Forty days prior to the current episode, the patient reported having experienced fever and a cough, and tested positive via a polymerase chain reaction (PCR) test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), from a nasopharyngeal swab. The latter symptoms, attributed to the viral infection, had completely resolved without the need for hospitalization. Informed consent was obtained. Our study follows the principles of the Declaration of Helsinki.
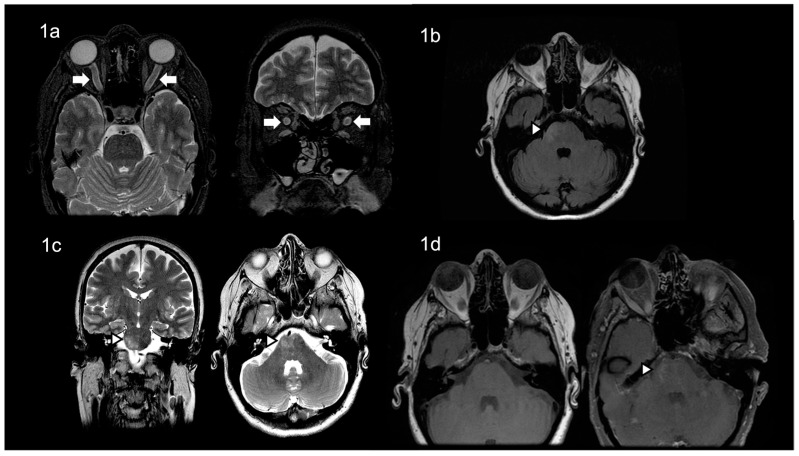
The ophthalmological examination revealed a decreased visual acuity (counting fingers) and a bilateral papilledema, while the rest of the neurological examination was unremarkable. A 3 Tesla MRI scan of the brain revealed a mild thickening of the bilateral optic nerves (Figure 1a) and high-intensity lesions in the medial and right lateral pons (Figure 1b–d).
Figure 1.
The patient’s brain MRI revealed, in T2 images, (a) mild thickening of the bilateral optic nerves, and (b,c) high-intensity lesions in the medial and right lateral pons, in T2/FLAIR images, (d) with contrast enhancement of the above lesions.
Routine blood tests presented no remarkable abnormalities, while serological studies with autoimmune markers, including antinuclear and anti-dsDNA antibodies, and the rheumatoid factor were not suggestive of other autoimmune diseases. A high titer of IgG and IgM antibodies against SARS-CoV-2 was detected in serum. An initial cerebrospinal fluid (CSF) analysis showed 7 cells/mm3 as well as normal protein (34.5 mg/dL) and glucose (50.9 mg/dL) levels. In addition, a CSF analysis with PCR for several viral and bacterial infections (Mumps virus, Measles virus, Human enterovirus, Parechovirus, Herpes simplex virus 1, Herpes simplex virus 2, Varicella zoster virus, Epstein–Barr virus, Cytomegalovirus, Human herpes virus 6, 7 and 8, Listeria monocytogenes, Heamophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus agalactiae, Neisseria meningitis, Borrelia burgdorferi, Escherichia coli K1, Cryptococcus neoformans, Cryptococcus gatii, West Nile virus, and SARS-CoV-2) was negative. The oligoclonal band status in CSF was negative. Furthermore, antibodies against myelin oligodendrocyte glycoprotein (anti-MOG) were revealed in serum (1/32) and CSF. According to the above, the diagnosis of anti-MOG brainstem encephalitis and optic neuritis was set.
Consequently, the patient was treated with 1000 mg of intravenous (IV) methylprednisolone for 5 days and oral phenytoin 4 mg/kg/day for 7 days as a retinoprotective agent [11]. Due to no significant clinical improvement, the patient additionally received intravenous immunoglobulin (IVIg) 2 g/kg, besides the oral prednisolone treatment. Following the treatment, the neurological examination showed no deficits, and the ophthalmological examination showed improvement in the visual acuity (6/10 in the right eye, 7/10 in the left eye) and a remission of the bilateral papilledema. The patient was therefore discharged with a prescription for oral prednisolone (30 mg/day).
3. Discussion
A comprehensive literature review of reported optic neuritis and COVID-19 infection cases was performed, including as many patients as possible. We used the PubMed database, with the following search terms: ‘’optic neuritis’’, ‘’MOG’’, ‘’encephalitis’’ and “COVID-19”. Additional articles were identified by hand-searching references of the included literature (Table 1).
Table 1.
Characteristics of patients with anti-MOG positive optic neuritis and anti-MOG encephalitis secondary to COVID-19 infection.
| Author (Year) | Age (Years)/Ethnicity/Sex (Male/Female) | Method of COVID-19 Diagnosis | COVID-19 Symptoms | Hospitalization (Yes/No) | Time of Onset of Neurological Manifestations from COVID-19 Diagnosis | Neurological Manifestations | MOG-Antibody Method of Detection | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| de Ruijter et al. (2020) | 15/Caucasian/Male | not confirmed | fever, nausea and cough | NM | Few weeks | Subacute vision loss with photopsias and frontal continuous headache |
NM | IVMP 1 g/day for three days | Symptoms resolved |
| Zhou et al. (2020) | 26/Hispanic/Male | nasal and oropharyngeal swabs (RT-PCR) |
dry cough | Yes | Few days | Bilateral, subacute, sequential vision loss first affecting the left eye, then the right eye 3 days later | MOG-IgG cell-based assays | IVMP 1 g/day for five days | Visual acuity improved rapidly |
| Sawalha et al. (2020) | 44/Hispanic/Male | nasopharyngeal swabs (RNA PCR) and serum (IgG abs) | shortness of breath and cough | No | Two weeks | Right eye pain that had progressed to his left eye along with bilateral blurring of vision leading to a complete vision loss | NM | IVMP 1 g/day for five days | Complete restoration of vision in the left eye with remarkable but not complete vision recovery in the right eye |
| Zoric et al. (2021) | 63/NM/Male | serology was positive for IgM and IgG antibodies against the virus | fatigue, shortness of breath, dry cough and fever | Yes | Four weeks | Right eye blurred vision | Indirect immunofluorescence (MOG antibodies) | IVMP 1 g/day for five days with prednisone tapering therapy for two weeks |
Visual acuity was improved, and disk edema was resolved entirely |
| Khan et al. (2021) | 11/NM/Male | nasopharyngeal swab was positive by CBNAAT | redness and ophthalmodynia in both eyes four days after a brief febrile illness |
Yes | Two weeks | Loss of vision in the right eye | NM | Pulse methylprednisolone with oral steroids continued and tapered over 12 weeks | Visual acuity was improved |
| Kogure et al. (2021) | 47/Japanese/Male | nasal and oropharyngeal swabs (PCR) |
asymptomatic | Yes | N/A | Left eye pain and an upper-visual-field defect | MOG-immunoglobulin G (MOG-IgG) testing in blood | IVMP 1 g/day for a total of 3 days, followed by an oral prednisolone tape | Visual acuity subsequently improved |
| Peters et al. (2021) | 23/NM/Male | nasopharyngeal PCR testing | asymptomatic | Yes | N/A | Progressive headache associated with dysesthesias fatigue, inattention, cognitive slowing, fevers, generalized seizures | MOG-IgG via FACS | IVMP 1 g/day for five days | Cognitive improvement |
| Durovic et al. (2021) | 22/NM/Male | PCR testing | severe headache, fever, neck stiffness, general weakness, and a loss of smell and taste | Ten days | Headache, neck rigidity | Serum MOG-IgG (live-cell assay) * | IVMP 1 g/day for five days | Symptoms resolved |
* Serum studies also revealed a low metabotropic glutamate receptor 1 (mGluR1) antibody titer (fixed cell assay, 1:40); CBNAAT, Cartridge Based Nucleic Acid Amplification Test; PCR, polymerase chain reaction; MOG, myelin oligodendrocyte glycoprotein; IVMP, intravenous methylprednisolone; NM, not mentioned; N/A, not applicable; FACS, Fluorescence-Activated Cell Sorting.
MOG antibody disease is mediated via antibodies against MOG, expressed on oligodendrocytes. MOG antibody disease is responsible for clinical manifestations like optic neuritis, transverse myelitis, encephalitis, and ADEM. Anti-MOG antibodies may be present in the bloodstream without causing symptoms until they enter the CNS. Along with lung cells, which are the main target of the SARS-CoV-2 virus, endothelial cells forming the blood–brain barrier (BBB) also express the angiotensin-converting enzyme 2 receptors (ACE2). By infecting these cells through ACE2 receptors, SARS-CoV-2 causes inflammation of the endothelium and the subsequent breakdown of the BBB. As a result, both anti-MOG and leukocytes can cross the BBB and trigger the onset of the disease [12,13].
Our case report adds to the existing bibliography on the neurological sequelae of COVID-19 [4]. With the ever-rising number of infected patients, it is reasonable to expect that these entities will also be encountered more frequently, and so clinicians must act promptly in diagnosing and treating patients. Therefore, a history of COVID-19 infection should raise awareness about these autoimmune and infection-triggered diseases, such as anti-MOG antibody disease.
4. Conclusions
Our case expands the spectrum of SARS-CoV-2 neurological disorders, since we here present a MOG-associated encephalitis and anti-MOG optic neuritis secondary to COVID-19 infection. While the incidence of these neuroimmune manifestations is low, early identification and initiation of corticosteroid therapy is essential to avoid disability. Despite the attempts of the international medical community to record all clinical manifestations of SARS-CoV-2, it seems that the emergence of new neuroimmunological manifestations after COVID-19 infection should raise awareness about MOG-related disease.
Author Contributions
Conceptualization, E.D.; methodology, Z.T. and A.P.; software, Z.T.; validation, Z.T., A.P., C.B., A.-M.A., V.S., V.T., N.G., G.M.H. and E.D.; investigation, Z.T., A.P., C.B., A.-M.A., V.S. and V.T.; writing—original draft preparation, Z.T.; writing—review and editing, Z.T., A.P., C.B., A.-M.A., V.S., V.T., N.G., G.M.H. and E.D.; supervision, E.D.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from the patient.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lima M., Siokas V., Aloizou A.M., Liampas I., Mentis A.A., Tsouris Z., Papadimitriou A., Mitsias P.D., Tsatsakis A., Bogdanos D.P., et al. Unraveling the Possible Routes of SARS-CoV-2 Invasion into the Central Nervous System. Curr. Treat. Options Neurol. 2020;22:37. doi: 10.1007/s11940-020-00647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novi G., Rossi T., Pedemonte E., Saitta L., Rolla C., Roccatagliata L., Inglese M., Farinini D. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020;7:e797. doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durovic E., Bien C., Bien C.G., Isenmann S. MOG antibody-associated encephalitis secondary to Covid-19: Case report. BMC Neurol. 2021;21:414. doi: 10.1186/s12883-021-02449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters J., Alhasan S., Vogels C.B.F., Grubaugh N.D., Farhadian S., Longbrake E.E. MOG-associated encephalitis following SARS-CoV-2 infection. Mult. Scler. Relat. Disord. 2021;50:102857. doi: 10.1016/j.msard.2021.102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Ruijter N.S., Kramer G., Gons R.A.R., Hengstman G.J.D. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: A case report. Mult. Scler. Relat. Disord. 2020;46:102474. doi: 10.1016/j.msard.2020.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou S., Jones-Lopez E.C., Soneji D.J., Azevedo C.J., Patel V.R. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Optic Neuritis and Myelitis in COVID-19. J. Neuroophthalmol. 2020;40:398–402. doi: 10.1097/WNO.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawalha K., Adeodokun S., Kamoga G.R. COVID-19-Induced Acute Bilateral Optic Neuritis. J. Investig. Med. High Impact Case Rep. 2020;8:2324709620976018. doi: 10.1177/2324709620976018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoric L., Rajovic-Mrkic I., Colak E., Miric D., Kisic B. Optic Neuritis in a Patient with Seropositive Myelin Oligodendrocyte Glycoprotein Antibody During the Post-COVID-19 Period. Int. Med. Case Rep. J. 2021;14:349–355. doi: 10.2147/IMCRJ.S315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan A., Panwala H., Ramadoss D., Khubchandani R. Myelin Oligodendrocyte Glycoprotein (MOG) Antibody Disease in a 11 Year Old with COVID-19 Infection. Indian J. Pediatr. 2021;88:488–489. doi: 10.1007/s12098-020-03656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kogure C., Kikushima W., Fukuda Y., Hasebe Y., Takahashi T., Shibuya T., Sakurada Y., Kashiwagi K. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis in a COVID-19 patient: A case report. Medicine. 2021;100:e25865. doi: 10.1097/MD.0000000000025865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raftopoulos R., Hickman S.J., Toosy A., Sharrack B., Mallik S., Paling D., Altmann D.R., Yiannakas M.C., Malladi P., Sheridan R., et al. Phenytoin for neuroprotection in patients with acute optic neuritis: A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:259–269. doi: 10.1016/S1474-4422(16)00004-1. [DOI] [PubMed] [Google Scholar]
- 12.Tsatsakis A., Calina D., Falzone L., Petrakis D., Mitrut R., Siokas V., Pennisi M., Lanza G., Libra M., Doukas S.G., et al. SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem. Toxicol. 2020;146:111769. doi: 10.1016/j.fct.2020.111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima M., Aloizou A.M., Siokas V., Bakirtzis C., Liampas I., Tsouris Z., Bogdanos D.P., Baloyannis S.J., Dardiotis E. Coronaviruses and their relationship with multiple sclerosis: Is the prevalence of multiple sclerosis going to increase after the COVID-19 pandemia? Rev. Neurosci. 2022;33:703–720. doi: 10.1515/revneuro-2021-0148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.