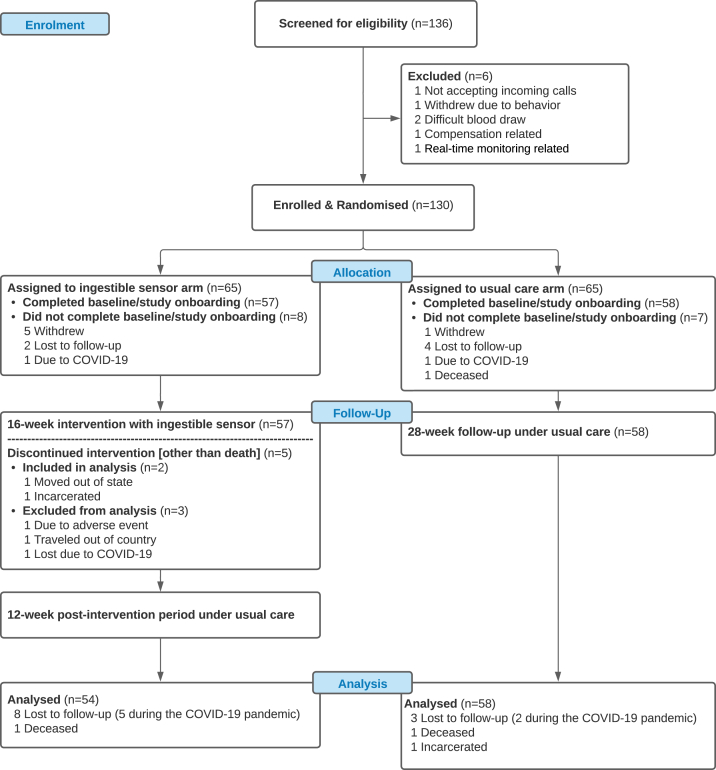
Fig. 1.
Recruitment and Randomisation of Patients. 136 HIV-infected individuals at least 18 years old with suboptimal ARV adherence at the time of screening were recruited in the main trial. We randomised 130 participants who met the inclusion criteria into IS group (N = 65) or UC group (N = 65). There were 57 participants in the IS group who completed baseline visits. One participant withdrew due to adverse. In UC group, there were 58 participants who completed their baseline visits. Two participants died from HIV progression during the study, none of which were thought to be study-related. A total of seven participants did not complete the study after baseline visits because of the COVID-19 pandemic (five in the IS group and two in the UC group). A total of 112 participants with at least one follow-up data collection after baseline were included in the final analysis with 54 in IS group and 58 in UC group.