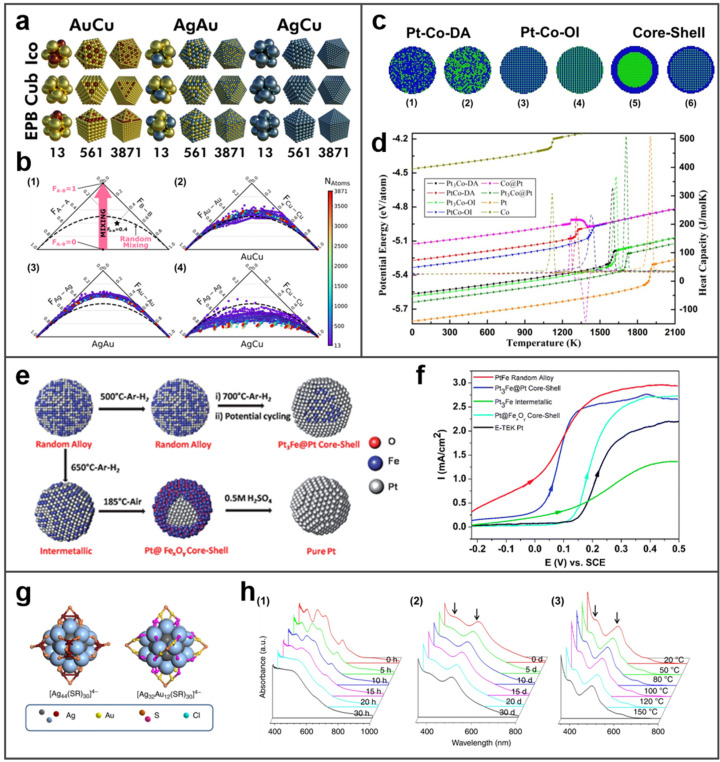
Figure 6.
The stability of BMNs. (a) The most stable chemical ordering and composition at the given size, shape, and metal pair. Reprinted with permission from Ref. [101]. (b) Bond composition plots: (1) guiding plot illustrating the theoretical mixing limits from no heteroatomic bonds (FA−B = 0) to no homoatomic bonds (FA−B = 1); (2) (3) and (4) the preferential compositions of AuCu, AgAu, and AgCu NPs at all sizes and structures in thermodynamics. Reprinted with permission from Ref. [101]. Copyright © 2020 American Chemical Society. (c) Schematic illustration of Pt–Co bimetallic NPs: (1) disordered Pt3–Co alloy (Pt3Co-DA), (2) disordered Pt–Co alloy (Pt–Co–DA), (3) ordered Pt3Co L12 intermetallics (Pt3Co–OI), (4) ordered PtCo L10 intermetallics (PtCo-OI), (5) Co–Pt core–shell structure (Co@Pt), and (6) Pt3Co–Pt core–shell structure (Pt3Co@Pt). Coloring denotes the type of atom: blue, Pt atom; green, Co atom. Adapted with permission from Ref. [103]. (d) Temperature dependence of potential energy and specific heat capacity for different Pt–Co bimetallic NPs (the dashed lines correspond to the heat capacity). Reprinted with permission from Ref. [103]. Copyright © 2017 American Chemical Society. (e) Pt–Fe bimetallic NPs with different architectures. Reprinted with permission from Ref. [104]. (f) Polarization curves on different Pt-Fe catalysts. Reprinted with permission from Ref. [104]. Copyright © The Royal Society of Chemistry 2011. (g) Structures of Ag NCs and Ag@Au NCs (the hydrocarbon tails and carboxylic groups of the protecting ligands are omitted). Reprinted with permission from Ref. [105]. (h) UV absorption spectra of (1) [Ag44(SR)30]4− and (2), (3) [Ag32Au12(SR)30]4− NCs (The arrows indicate the absorption features at 390 and 490 nm). Reprinted with permission from Ref. [105]. Copyright © 2022 Springer Nature Limited.