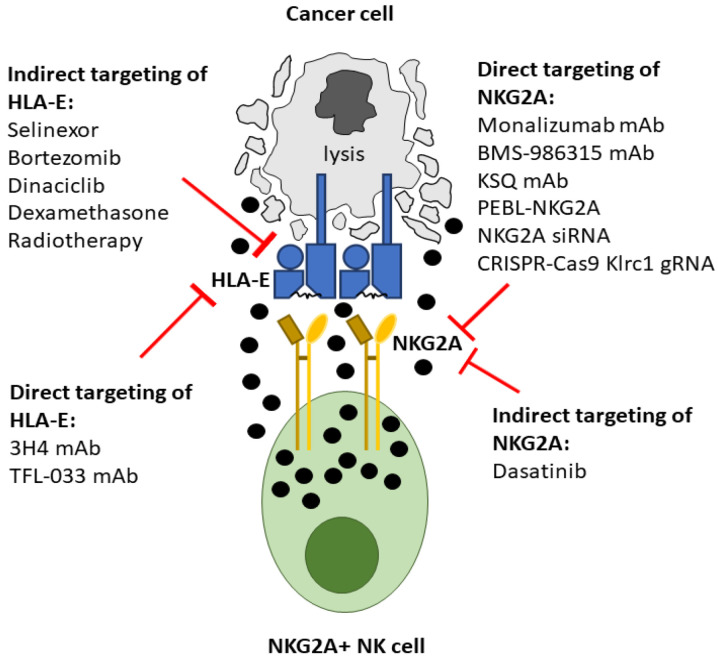
Figure 2.
Therapeutic strategies to disrupt the inhibitory NKG2A:HLA-E axis and promote NK cell activation against malignant cells. Blockade of NKG2A:HLA-E interactions enhances cytotoxic granule release and cytokine production by NKG2A+ NK cells resulting in cancer cell lysis. Monoclonal antibodies (mAb) against HLA-E or NKG2A directly modulate NK cell effector functions by inhibiting the interaction of HLA-E and NKG2A. Modulation of NKG2A expression on NK cells also promotes NK cell anti-tumour functions. Expression of NKG2A at the plasma membrane can be downregulated by protein expression blockers (NKG2A-PEBL) which specifically constrain NKG2A in the Golgi. Genetic disruption to the NKG2A locus (Klrg1) can be achieved with CRISPR-Cas9 technology which inhibits its expression. Dasatinib, a tyrosine kinase inhibitor, also downregulates NKG2A expression leading to enhanced NK cell activity against tumours. Downregulation of surface HLA-E on tumour cells can be achieved with multiple different drugs that inhibit a variety of pathways. These include selinexor, a selective inhibitor of nuclear export; bortezomib, a proteasome inhibitor; dinaciclib, a pan-CDK inhibitor and dexamethasone, an activator of glucocorticoid receptors. Radiotherapy, which produces DNA damage can alter HLA-E expression on cancer cells.