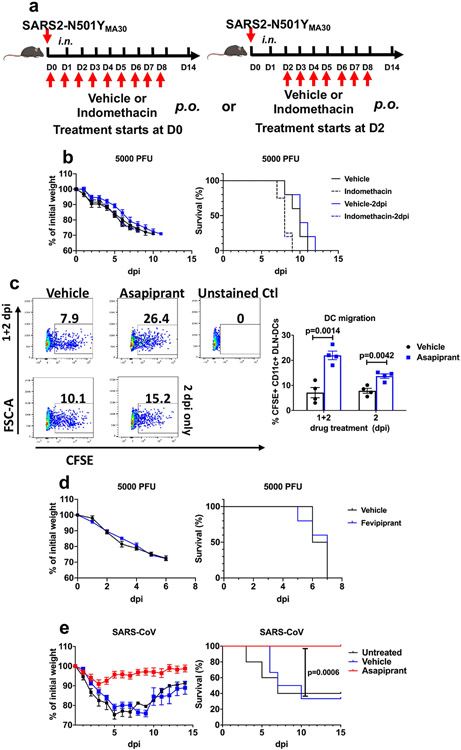
Extended Data Fig. 6 ∣. Effects of asapiprant, indomethacin and fevipiprant in aged mice infected with SARS-CoV-2 or SARS-CoV.
a, Schematic showing experimental design for results shown in panel b (mouse image with BioRender. com). b, Percentage of initial weight and survival of middle-aged C57BL/6 mice infected with 5000 PFU of SARS2-N501YMA30 after indomethacin treatment (n = 5 in all groups except in the group when indomethacin was given at 0 dpi, where n = 4). c, Middle-aged C57BL/6 mice were instilled with CFSE intranasally 6 h before infection with 5000 PFU of SARS2-N501YMA30. One group of mice received vehicle or asapiprant at 1 and 2 dpi (1+2) while another group of mice received drug treatment only at 2 dpi (2). Mice were euthanized at 3 dpi and lung draining lymph node (DLN; mediastinal) were harvested and analyzed with flow cytometry. rDC migration from the lung to DLN was measured as the frequency of CFSE+CD11c+ cells in the DLN (n = 4). d, Percentage of initial weight and survival of middle-aged C57BL/6 mice infected with 5000 PFU of SARS2-N501YMA30 with fevipiprant (CRTH2 inhibitor) treatment (two times a day; days 2 to 8; 5 mg/kg) (n = 4, vehicle; n = 5, fevipiprant). e, Percentage of initial weight and survival of middle-aged C57BL/6 mice infected with 104 PFU of SARS-CoV, with vehicle or asapiprant treatment from day 2 to 8 (n = 5, untreated; n = 12, vehicle and asapiprant). P values determined by two-tailed Student’s t test in a and log-rank (Mental-Cox) in e. Data are mean ± s.e.m. and are representative of at least two independent experiments.