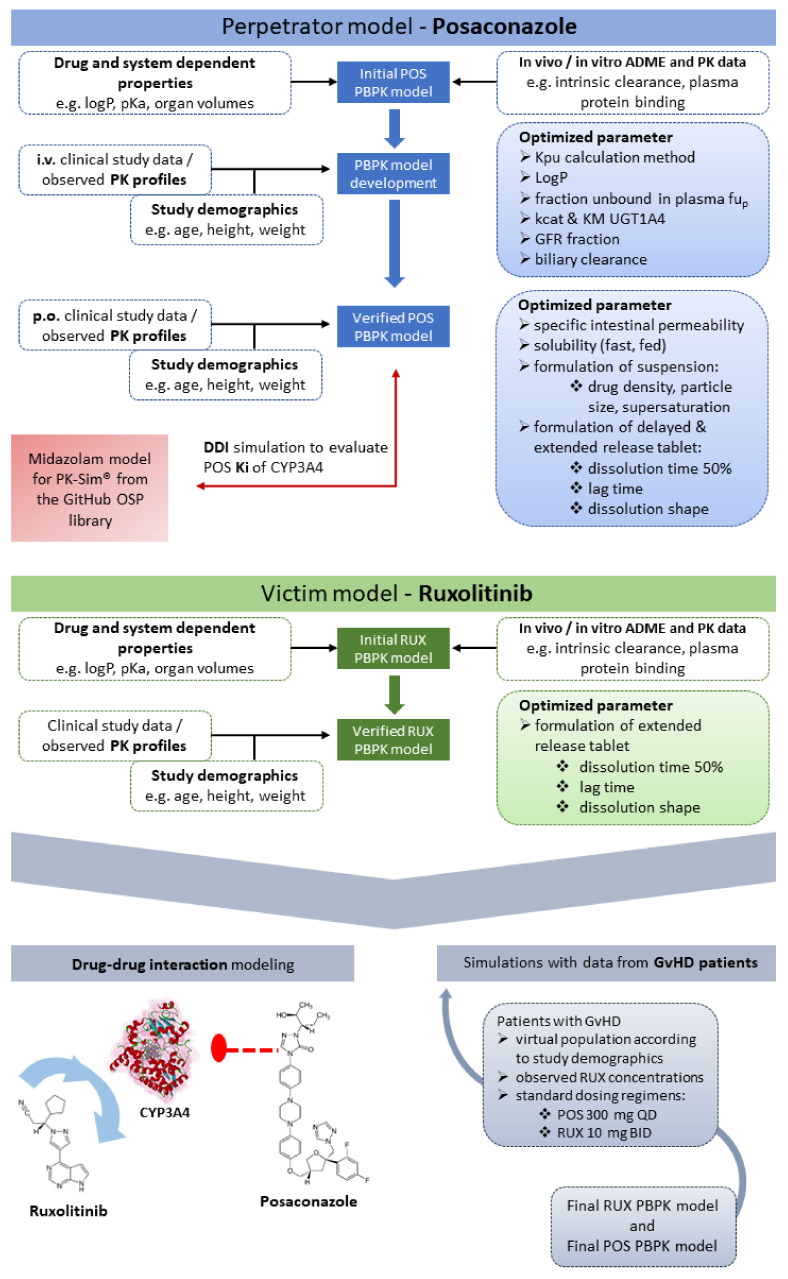
Figure 1.
Schematic workflow showing the development and evaluation of the perpetrator (POS) and victim (RUX) PBPK models, which were developed separately. Initial model development started with basic input parameters from the literature. Parameters optimized during model building are shown in the blue and green rectangles, respectively. The final input parameters are given in Tables S2 and S6. The entire model building and evaluation process was supported using clinical study data, used either as training or test dataset (see Tables S1 and S5 for allocation). The final POS and RUX models were applied to simulate DDI between these substances and simulations with data obtained from GvHD patients were conducted (see gray rectangle).