Figure 1.
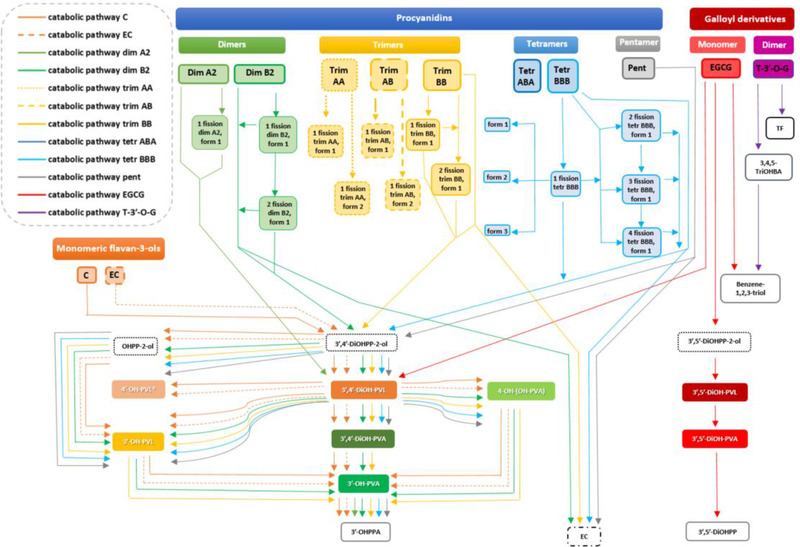
Proposed catabolic pathways and catabolites produced after in vitro faecal fermentation of monomeric and oligomeric flavan‐3‐ols. C: (+)‐catechin; EC: (−)‐epicatechin; Dim: dimer; Trim: trimer; Tetr: tetramer; Pent: pentamer; EGCG: (−)‐epigallocatechin‐3‐O‐gallate; T‐3′‐O‐G: theaflavin‐3′‐O‐gallate; OHPP‐2‐ol: 1‐(hydroxyphenyl)‐3‐(2″,4″,6″‐trihydroxyphenyl)‐propan‐2‐ol; 3′,5′‐DiOHPP‐2‐ol: 1‐(3′,5′‐dihydroxyphenyl)‐3‐(2″,4″,6″‐trihydroxyphenyl)‐propan‐2‐ol; 3′,4′‐DiOHPP‐2‐ol: 1‐(3′,4′‐dihydroxyphenyl)‐3‐(2″,4″,6″‐trihydroxyphenyl)‐propan‐2‐ol; 3′‐OH‐PVL: 5‐(3′‐hydroxyphenyl)‐γ‐valerolactone; 4′‐OH‐PVL: 5‐(4′‐hydroxyphenyl)‐γ‐valerolactone; 3′,5′‐DiOH‐PVL: 5‐(3′,5′‐dihydroxyphenyl)‐γ‐valerolactone; 3′,4′‐DiOH‐PVL: 5‐(3′,4′‐dihydroxyphenyl)‐γ‐valerolactone; 3′‐OH‐PVA: 5‐(3′‐hydroxyphenyl)valeric acid; 4‐OH‐(OH‐PVA): 4‐hydroxy‐5‐(hydroxyphenyl)valeric acid; 3′,5′‐DiOH‐PVA: 5‐(3′,5′‐dihydroxyphenyl)valeric acid; 3′,4′‐DiOH‐PVA: 5‐(3′‐4′‐dihydroxyphenyl)valeric acid; 3′‐OHPPA: 3‐(3′‐hydroxyphenyl)propanoic acid; 3′,5′‐DiOHPPA: 3‐(3′,5′‐dihydroxyphenyl)propanoic acid; 3,4,5‐TriOHBA: 3,4,5‐trihydroxybenzoic acid; TF: theaflavin. * means not quantified in fermented samples.
