Figure 2.
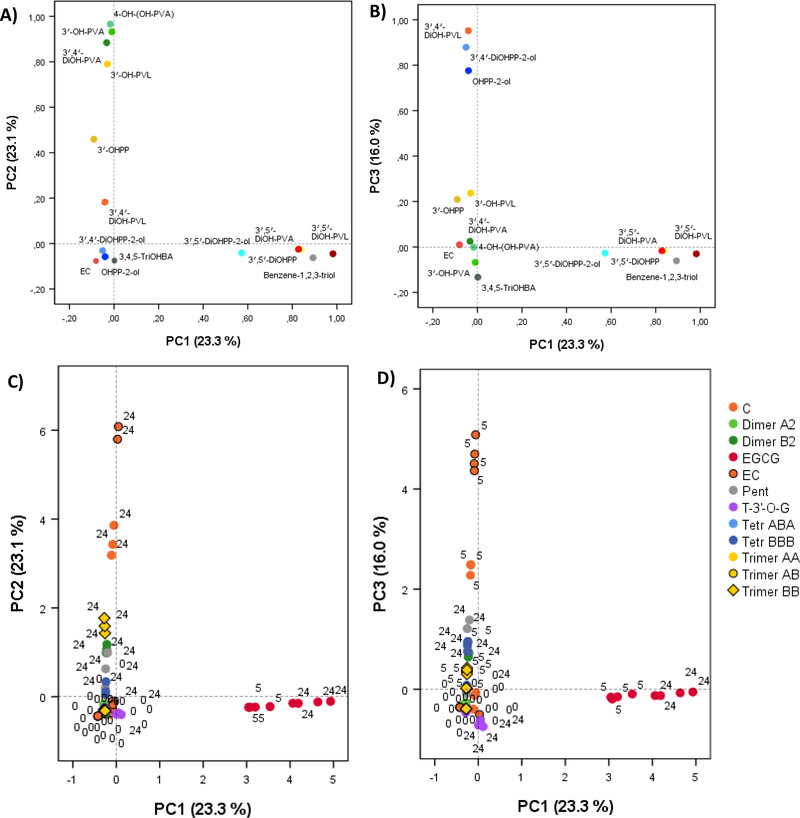
PCA to explore differences in the behavior of parent compounds in the in vitro colonic environment and in the appearance of catabolites over the faecal fermentation of monomeric and oligomeric flavan‐3‐ols. Loading plots of PC1 versus PC2 (A), PC1 versus PC3 (B). Score plots of the concentrations of identified catabolites obtained from PC1 and PC2 (C), PC1 and PC3 (D). The number accounts for the collection time (0, 5, and 24 h). C: (+)‐catechin; EC: (−)‐epicatechin; Dim: dimer; Trim: trimer; Tetr: tetramer; Pent: pentamer; EGCG: (−)‐epigallocatechin‐3‐O‐gallate; T‐3′‐O‐G: theaflavin‐3′‐O‐gallate. OHPP‐2‐ol: 1‐(hydroxyphenyl)‐3‐(2″,4″,6″‐trihydroxyphenyl)‐propan‐2‐ol; 3′,5′‐DiOHPP‐2‐ol: 1‐(3′,5′‐dihydroxyphenyl)‐3‐(2″,4″,6″‐trihydroxyphenyl)‐propan‐2‐ol; 3′,4′‐DiOHPP‐2‐ol: 1‐(3′,4′‐dihydroxyphenyl)‐3‐(2″,4″,6″‐trihydroxyphenyl)‐propan‐2‐ol; 3′‐OH‐PVL: 5‐(3′‐hydroxyphenyl)‐γ‐valerolactone; 3′,5′‐DiOH‐PVL: 5‐(3′,5′‐dihydroxyphenyl)‐γ‐valerolactone; 3′,4′‐DiOH‐PVL: 5‐(3′,4′‐dihydroxyphenyl)‐γ‐valerolactone; 3′‐OH‐PVA: 5‐(3′‐hydroxyphenyl)valeric acid; 4‐OH‐(OH‐PVA): 4‐hydroxy‐5‐(hydroxyphenyl)valeric acid; 3′,5′‐DiOH‐PVA: 5‐(3′,5′‐dihydroxyphenyl)valeric acid; 3′,4′‐DiOH‐PVA: 5‐(3′,4′‐dihydroxyphenyl)valeric acid; 3′‐OHPPA: 3‐(3′‐hydroxyphenyl)propanoic acid; 3′,5′‐DiOHPPA: 3‐(3′,5′‐dihydroxyphenyl)propanoic acid; 3,4,5‐TriOHBA: 3,4,5‐trihydroxybenzoic acid. Too specific compounds as fission catabolites from oligomers were not included.
