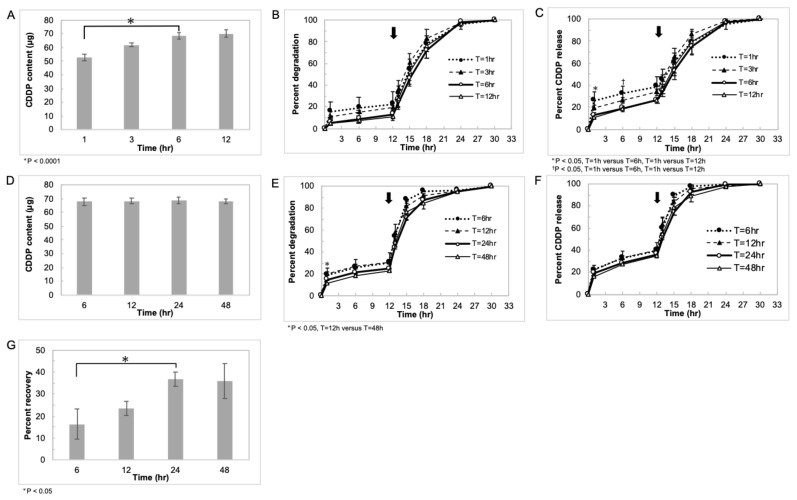
Figure 3.
Characterization of CCGH prepared by varying the preparation conditions in vitro. (A–C) CDDP content per 1 mg CCGH (A), degradation profiles (B), and CDDP release profiles (C) of CCGH prepared by varying the stirring time of the gelatin aqueous solution and the CDDP aqueous solution at 40 °C (1 h; dotted line, 3 h; dashed line, 6 h; thick solid line, and 12 h; thin solid line) (n = 3). Data were represented as mean ± SD. Collagenase D was added at the time indicated by the arrow (12 h). (D–G) CDDP content per 1 mg CCGH (D), degradation profiles (E), CDDP release profiles (F), and recovery rate (G) of CCGH prepared by varying the standing time of the stirred solution at 4 °C (6 h; dotted line, 12 h; dashed line, 24 h; thick solid line, and 48 h; thin solid line). (n = 3). Data were represented as mean ± SD. Collagenase D was added at the time indicated by the arrow (12 h).