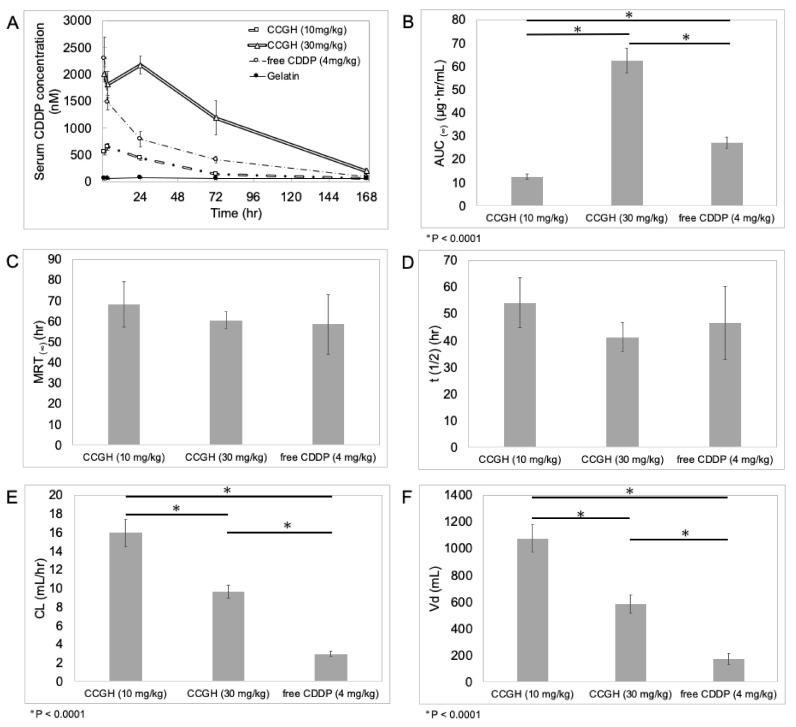
Figure 5.
In vivo retention evaluation of CDDP after intraperitoneal administration. (A) Serum CDDP concentration profiles after intraperitoneal administration of free CDDP solution, CCGH, and gelatin solution. CCGH (10 mg/kg; double dot-dash line), CCGH (30 mg/kg; double line), free CDDP solution (4 mg/kg; dot-dash line), and gelatin solution (thin solid line). (n = 5). Data were represented as mean ± SD. (B–F) Main pharmacokinetic parameters of CCGH (10, 30 mg/kg) and CDDP (4 mg/kg). (n = 5). (B) AUC, area under the serum concentration–time curve; (C) MRT, mean residence time; (D) t (1/2), elimination half-life; (E) CL, clearance; (F) Vd, distribution volume.