Summary
Rapid eye movement (REM) obstructive sleep apnea might be particularly harmful to the cardiovascular system. We aimed to investigate the association between sleep apnea during REM sleep and signs of atherosclerotic disease in the form of carotid intima thickness in a community‐based sample of men and women and possible sex differences in this association. The association between sleep apnea during REM sleep and intima thickness was analysed cross‐sectionally in women from the community‐based “Sleep and Health in Women” (SHE) study (n = 253) and age‐ and body mass index (BMI)‐matched men from the “Men in Uppsala; a Study of sleep, Apnea and Cardiometabolic Health” (MUSTACHE) study (n = 338). Confounders adjusted for were age, BMI, gender, alcohol, and smoking. All participants underwent a full‐night polysomnography, high‐resolution ultrasonography of the common carotid artery, anthropometric measurements, blood pressure measurements, and answered questionnaires. There was an association between sleep apnea during REM sleep and thicker carotid intima that remained after adjustment for confounding (adjusted β = 0.008, p = 0.032). The intima was increased by 9.9% in the group with severe sleep apnea during REM sleep, and this association between severe sleep apnea during REM sleep and increased intima thickness remained after adjustment for confounders (adjusted β = 0.043, p = 0.021). More women than men had severe sleep apnea during REM sleep; moreover, in sex‐stratified analyses, the association between sleep apnea during REM sleep and intima thickness was found in women but not in men. We conclude that severe REM sleep apnea is independently associated with signs of atherosclerosis. When stratified by sex, the association is seen in women but not in men.
Keywords: atherosclerosis, gender difference, REM sleep, sleep‐disordered breathing
1. INTRODUCTION
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular disease (CVD) (Marin et al., 2005; Redline et al., 2010). The standard method for quantifying the severity of OSA is to measure the number of apneas and hypopneas per hour of sleep. However, OSA is a heterogeneous disorder with physiological and polysomnographic differences between groups and by sex (Won et al., 2020). Hence, there is an urgent need to identify the OSA phenotype that is most important when it comes to cardiovascular consequences. Some previous studies have indicated that the negative effect of OSA might differ by sleep stages, and that the physiological effects are most pronounced when apneas and hypopneas appear during the rapid eye movement (REM) sleep stage (Appleton et al., 2016; Chen et al., 2014; Ljunggren et al., 2020; Mokhlesi et al., 2014).
Rapid eye movement sleep is a stage of vivid dreaming, variable respiration, and bursts of sympathetic activity (Somers et al., 1993). Rapid eye movements appear in ~20% of sleep and is especially seen in the morning hours of sleep (Sahlin et al., 2009). The electroencephalogram is similar to the awake activity, and the breathing frequency is increased. However, the muscles are paralysed, with reduced activity in the chin‐electromyogram, rib muscles, and the genioglossus (Aserinsky & Kleitman, 1953; Eckert et al., 2009; McSharry et al., 2014). The blood pressure is high during REM sleep, especially during the sudden bursts of sympathetic activity in the phasic REM sleep (Somers et al., 1993).
Obstructive sleep apnea is aggravated during REM sleep with longer apneas, and more severe hypoxic events (Findley et al., 1985). Paradoxically, women have a greater proportion of apneas during REM sleep, despite OSA occurring less often during a whole night sleep in women than in men (O'Connor et al., 2000; Won et al., 2020). REM‐OSA is related to arterial hypertension and non‐dipping blood pressure (Appleton et al., 2016; Mokhlesi et al., 2014, 2015).
Carotid artery intima thickness is a marker of atherosclerosis and can detect early, sub‐clinical changes (Akhter et al., 2013; Rodriguez‐Macias et al., 2006; M. Xu et al., 2021). From the “Sleep and Health in Women” (SHE), we reported that severe REM‐OSA was independently and prospectively related to intima thickness, while severe OSA based on all sleep stages, that is, total sleep time (TST), was not (Ljunggren et al., 2018).
The aim of the present study was to investigate the association between REM‐OSA and carotid intima thickness in a community‐based sample of men and women and to investigate possible sex differences in this association. To achieve this, we have created an age‐ and body mass index (BMI)‐matched community‐based cohort of women and men.
2. METHODS
2.1. Study population
The study population comprised women from the follow‐up study in the community‐based cohort study SHE (n = 400) (Ljunggren et al., 2020) and age and BMI matched men from the “Men in Uppsala; a Study of sleep, Apnea and Cardiometabolic Health” (MUSTACHE) study (n = 400) (Figure 1).
FIGURE 1.
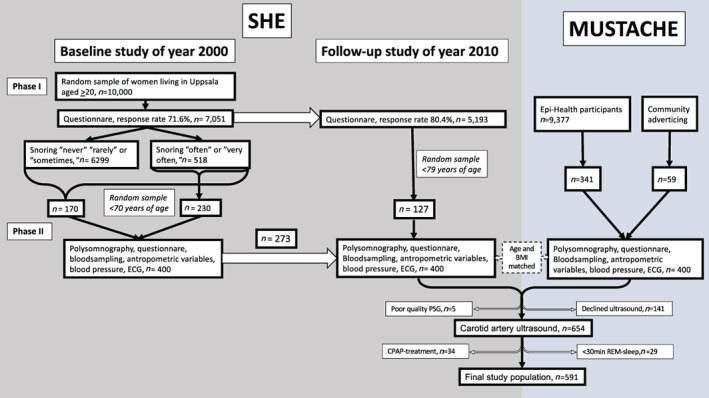
Flow chart describing the “Sleep and Health in Women” (SHE) and “Men in Uppsala; a Study of sleep, Apnea and Cardiometabolic Health” (MUSTACHE) cohorts and the final study sample
The SHE study was initiated in the year 2000, with a postal questionnaire sent to randomly selected women from the population registry of the City of Uppsala, Sweden. Of the total study population aged 20–70 years (n = 6,112), a random sample of 400 women, with an oversampling of snorers, participated in Phase II of the study and was investigated with a whole night polysomnography (PSG), questionnaires, anthropometric measurements, blood sampling, blood pressure measurement, and an electrocardiogram during 2001–2004 (Ljunggren et al., 2012). A follow‐up study was initiated in 2010 with a postal questionnaire to the whole cohort, and the participants from Phase II were invited to perform a new PSG. Out of the 400 original participants, 273 accepted. An additional 127 women, randomly selected from the participants answering the follow‐up questionnaire, also participated in the second phase of the follow‐up study that was performed during 2012–2014.
The MUSTACHE study was initiated in 2016 when men participating in the community‐based study Epi‐Health (Lind et al., 2013) during 2011–2015 (n = 9,377), matched with the women in the SHE cohort by age and BMI, were recruited. If no match could be recruited among the Epi‐Health participants (n = 59), a matching man was recruited from the community by local announcements. The men were investigated with a whole night PSG, questionnaires, anthropometric measurements, blood sampling, and blood pressure measurement using the same protocol as for the SHE study.
All women in the follow‐up study in SHE who had PSG, and all men in the MUSTACHE study were asked to undergo high‐resolution ultrasonography of the common carotid artery. We have previously published longitudinal data, using baseline PSG and carotid data from the follow‐up for the women in the SHE study (Ljunggren et al., 2018). For the present study, cross‐sectional data were used for both the men and the women from the follow‐up study. Participants with continuous positive airway pressure (CPAP)‐treated OSA (n = 34) or <30 min of REM sleep (n = 29) were excluded.
2.2. Ethics statement
Written informed consent was obtained from all the participants, and the Ethics Committee at Uppsala University, Uppsala, Sweden, approved the study protocol (approval number 2009/379 and 2016/029).
2.3. Assessment of sleep disordered breathing
All participants underwent whole‐night ambulatory PSG (EMBLA, Flaga Inc., Iceland) using the same equipment in the SHE and the MUSTACHE studies (Ljunggren et al., 2020). Sleep was then scored manually by the same investigator, and with identical scoring criteria, in both cohorts. For details on scoring criteria, see the online data supplement.
Commonly used cut‐offs for the apnea‐hypopnea index (AHI) were used to categorise OSA in no (<5 events/h), mild (5 to <15 events/h), moderate (15 to <30 events/h), and severe (≥30 events/h) disease. The same cut‐offs were used for the oxygen desaturation index (ODI) and REM‐AHI.
2.4. Assessment of covariates
The participants answered questionnaires including questions on daytime symptoms, lifestyle factors such as smoking and alcohol consumption, and medical history and medication. Additional details on covariates and definitions are provided in the online data supplement.
The morning after the PSG, fasting blood samples were drawn for the analysis of C‐reactive protein (CRP) and low‐density lipoprotein (LDL). Blood pressure was measured in the right arm in a supine position after 15 min of rest. The participants’ height and weight were measured, and the BMI was calculated.
2.5. Carotid artery ultrasonography
The participants were examined with high‐resolution ultrasonography (Collagenoson Minhorst Company, Meudt, Germany) of the left common carotid artery to assess the intima thickness. The same investigators performed and analysed the ultrasonograms in both the SHE and the MUSTACHE cohort. The investigators were blinded to the PSG results. The method has been described in detail previously (Rodriguez‐Macias et al., 2006), and information is also provided in the online data supplement.
2.6. Statistical methods
Statistical analyses were performed using Stata 16.1 (Stata Corporation, College Station, TX, USA). Associations between REM‐OSA and carotid artery intima thickness were analysed using linear regression with log‐transformed intima thickness as a dependent variable, first with log‐transformed REM‐AHI as a continuous variable and second with REM‐AHI as a categorical variable. The associations were analysed first in an age adjusted model; second in a model adjusting for confounding by age, BMI, alcohol, smoking, and sex (Model 1); and finally, in a model considering possible intermediate mechanisms also including systolic blood pressure, LDL, CRP, and diabetes (Model 2). Data that were not normally distributed were log transformed, and the validity of the regression models were examined with regression diagnostics. The analyses were further stratified by sex, and interaction based on sex was investigated. The analyses were also repeated in the sub‐sample of individuals with an AHI <15 events/h and non‐REM‐AHI <5 events/h. For comparison, associations between other measures of OSA (AHI, ODI, and proportion of TST with saturation <90%) and carotid artery intima thickness were analysed in the same way.
3. RESULTS
3.1. Population characteristics
The final study population consisted of 338 men and 253 women (Figure 1). In total, 157 participants (26.6%) had severe REM‐OSA defined as REM‐AHI ≥30 events/h, while 59 participants (10.0%) had severe OSA defined as AHI ≥30 events/h.
Baseline characteristics and PSG parameters for the whole study population and by severity of REM‐OSA are presented in Table 1; characteristics by AHI below or above 30 events/h can be found in Table S1.
TABLE 1.
Baseline characteristics and polysomnographic data for the participants
| Characteristic | All | REM AHI <30 events/h | REM AHI ≥30 events/h |
|---|---|---|---|
| No. of subjects | 591 | 434 | 157 |
| Women, n (%) | 253 (42.8) | 157 (36.2) | 96 (61.2) |
| Age, years, mean (SD) | 59.3 (11.1) | 57.5 (11.3) | 64.4 (8.5) |
| Body mass index, kg/m2, mean (SD) | 26.2 (4.1) | 25.6 (3.7) | 27.9 (4.7) |
| Smoking status, n (%) | |||
| Never | 341 (57.8) | 264 (60.8) | 77 (49.4) |
| Former | 213 (36.1) | 145 (33.4) | 68 (43.6) |
| Current | 36 (6.1) | 25 (5.8) | 11 (7.1) |
| Alcohol status, n (%) | |||
| Do not drink | 53 (9.0) | 40 (9.2) | 13 (8.3) |
| CAGE <2 points | 459 (77.7) | 336 (77.4) | 123 (78.3) |
| CAGE ≥2 points | 63 (10.7) | 44 (10.1) | 19 (12.1) |
| Hypertension, n (%) | 141 (24.1) | 85 (19.6) | 56 (36.6) |
| Antihypertensive drugs, n (%) | 164 (27.8) | 93 (21.4) | 71 (45.2) |
| Lipid lowering drugs, n (%) | 67 (11.3) | 46 (10.6) | 21 (13.4) |
| Diabetes, n (%) | 30 (5.2) | 18 (4.2) | 12 (7.7) |
| Systolic blood pressure, mmHg, mean (SD) | 131.0 (15.8) | 129.6 (15.5) | 135.0 (15.9) |
| Epworth Sleepiness scale, points, mean (SD) | 7.3 (4.2) | 7.1 (4.2) | 7.6 (4.2) |
| LDL, mmol/L, mean (SD) | 3.5 (0.9) | 3.4 (0.9) | 3.7 (0.9) |
| CRP, mg/L, mean (SD) | 1.1 (0.6–2.2) | 1.0 (0.5–2.0) | 1.4 (0.7–2.5) |
| TST, min, mean (SD) | 394.3 (60.5) | 394.9 (62.2) | 392.7 (55.7) |
| AHI, events/h, median (IQR) | 6.5 (1.6–16.4) | 3.5 (1.0–8.2) | 22.5 (15.7–35.4) |
| ODI 3, events/h, median (IQR) | 7.7 (2.2–18.4) | 4.4 (1.4–9.8) | 23.3 (15.9–33.2) |
| ODI 4, events/h, median (IQR) | 4.1 (0.8–11.6) | 2.1 (0.4–5.7) | 16.0 (9.2–25.9) |
| Non‐REM AHI, events/h, median (IQR) | 4.4 (1.0–13.2) | 2.6 (0.6–6.9) | 16.9 (10.2–32.6) |
| REM AHI, events/h, median (IQR) | 10.4 (1.9–31.4) | 4.4 (1.2–14.3) | 42.4 (35.6–51.1) |
| Saturation, %, mean (SD) | 94.2 (1.7) | 94.4 (1.6) | 93.4 (1.7) |
| Lowest saturation, %, median (IQR) | 86.0 (82.0–90.0) | 88.0 (84.0–91.0) | 81.0 (77.0–85.0) |
| Proportion of TST with saturation <90%, %, median (IQR) | 0.4 (0.0–2.8) | 0.1 (0.0–1.2) | 2.9 (0.8–9.3) |
| Proportion of TST with REM sleep, % | 19.4 (5.0) | 19.8 (5.1) | 18.4 (4.8) |
| Intima thickness, mm, median (IQR) | 0.08 (0.07–0.09) | 0.08 (0.07–0.09) | 0.09 (0.08–0.10) |
AHI, apnea‐hypopnea index; CRP, C‐reactive protein; IQR, interquartile range; LDL, low‐density lipoprotein; ODI, oxygen desaturation index; REM, rapid eye movement; TST, total sleep time.
Participants with severe sleep disordered breathing, defined either as REM‐AHI ≥30 events/h or AHI ≥30 events/h, were older, had a higher BMI, and more often a diagnosis of hypertension than participants with REM‐AH or AHI <30 events/h, respectively.
The mean age and BMI did not differ between men and women (Table S2). Systolic blood pressure and prevalence of hypertension and diabetes were also similar in men and women. Smoking and alcohol habits differed by gender; women were more often former or current smokers, while alcohol overconsumption was more common in men. For the PSG parameters, there were several sex differences. Women had a longer TST, while there was no difference in REM sleep percentage. Women also had a slightly higher AHI, with a median (interquartile range) AHI of 8.7 (2.5–19.5) events/h compared to 5.1 (1.3–14.0) events/h in men. With AHI divided by sleep stage, the sex difference was more pronounced for REM‐AHI (17.6 events/h in women and 6.5 events/h in men) than for non‐REM‐AHI (5.2 events/h in women and 3.9 events/h in men). Also, severe REM‐OSA was more common in women (37.9%) than in men (18%). Despite the higher AHI, women had a better Mean (SD) saturation during the night at 94.7% (1.7%) versus 93.8% (1.6%).
3.2. Carotid intima thickness
There was an association between REM‐OSA and thicker carotid intima. The association remained after adjustment for confounding by age, BMI, smoking, alcohol, and sex (Table 2), and also after further adjustment for the possible intermediators: systolic blood pressure, LDL, CRP, and diabetes (adjusted β = 0.010, p = 0.006). OSA assessed by the AHI was associated with intima thickness in unadjusted models. However, after adjustment for age, BMI, smoking, alcohol, and sex, the association was no longer significant (Table 2). There was no association between ODI and intima thickness. Proportion of TST with saturation <90% was associated with intima thickness; (adjusted β = 0.0097, p = 0.007) after adjustment for age, BMI, alcohol, smoking and gender. When proportion of TST with saturation <90% was added to the multivariate model for REM‐AHI, there was a significant association between REM‐AHI and intima thickness (adjusted β = 0.0079, p = 0.049), while not for proportion of TST with saturation <90% (adjusted β = 0.0063, p = 0.114).
TABLE 2.
Associations between different measures of sleep disordered breathing and intima thickness
| Sleep‐disordered breathing and intima thickness | ||||||
|---|---|---|---|---|---|---|
| Age‐adjusted | Adjusted for age, BMI, alcohol, smoking, and sex | |||||
| β‐coef. | (95% CI) | p | β‐coef. | (95% CI) | p | |
| REM‐AHI | 0.010 | (0.004 to 0.017) | 0.002 | 0.008 | (0.001 to 0.015) | 0.032 |
| AHI | 0.009 | (0.001 to 0.017) | 0.028 | 0.006 | (−0.002 to 0.015) | 0.155 |
| ODI 3 | 0.007 | (−0.002 to 0.015) | 0.127 | 0.004 | (−0.006 to 0.013) | 0.439 |
AHI, apnea‐hypopnea index; CI, confidence interval; coef., coefficient; ODI, oxygen desaturation index; REM, rapid eye movement.
Results from the linear regression analysis with intima thickness, REM‐AHI, AHI, and ODI log transformed. Each line in the table represents a separate regression model.
When analysing the different categories of REM‐OSA, the intima was increased by 9.9% in the group with severe REM‐OSA compared to those without REM‐OSA. In adjusted models, an association was seen for severe REM‐OSA and intima thickness, but not for milder REM‐OSA (Table 3). Adding lipid‐lowering drugs to the confounders in Model 2 did not affect the significant findings.
TABLE 3.
Associations between severity of sleep apnea during rapid eye movement (REM) sleep‐obstructive sleep apnea (OSA) and intima thickness
| Severity of REM‐OSA and intima thickness | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| REM‐AHI, events/h | N | Median (IQR) | Age‐adjusted | Adjusted Model 1 | Adjusted Model 2 | ||||||
| β‐coef. | (95% CI) | p | β‐coef. | (95% CI) | p | β‐coef. | (95% CI) | p | |||
| 0–4.9 | 222 | 0.08 (0.07–0.09) | Ref | Ref | Ref | ||||||
| 5–14.9 | 113 | 0.08 (0.08–0.09) | 0.034 | −0.002 to 0.069 | 0.062 | 0.032 | −0.004 to 0.068 | 0.085 | 0.041 | 0.004 to 0.078 | 0.029 |
| 15–29.9 | 99 | 0.08 (0.08–0.09) | 0.021 | −0.017 to 0.059 | 0.275 | 0.015 | −0.025 to 0.054 | 0.468 | 0.026 | −0.015 to 0.068 | 0.212 |
| ≥30 | 157 | 0.09 (0.08–0.10) | 0.058 | 0.025 to 0.092 | 0.001 | 0.043 | 0.006 to 0.080 | 0.021 | 0.055 | 0.017 to 0.092 | 0.005 |
AHI, apnea‐hypopnea index; CI, confidence interval; coef., coefficient; IQR, interquartile range; OSA, obstructive sleep apnea; REM, rapid eye movement.
Results from linear regression analysis with log‐transformed intima thickness as dependent variable. Model 1 adjusted for age, BMI, alcohol, smoking, and sex. Model 2 adjusted for age, body mass index, alcohol, smoking, sex, systolic blood pressure, low‐density lipoprotein, C‐reactive protein, and diabetes.
In sex‐stratified analyses, an association was observed between REM‐OSA and intima thickness in women, but not in men (Table 4); however, there was no significant interaction based on sex (p = 0.493). Moreover, an association was seen between AHI and intima thickness in women (adjusted β = 0.017, p = 0.039 in Model 1), but not in men (adjusted β = 0.002, p = 0.681) (Table S3).
TABLE 4.
Associations between sleep apnea during REM‐sleep (REM‐OSA) and intima thickness in men and women
| REM‐OSA and intima thickness in men and women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median (IQR) | Age‐adjusted | Adjusted Model 1 | Adjusted Model 2 | |||||||
| β‐coef. | (95% CI) | p | β‐coef. | (95% CI) | p | β‐coef. | (95% CI) | p | |||
| Men REM‐AHI, events/h | |||||||||||
| 0–4.9 | 151 | 0.08 (0.07–0.09) | Ref | Ref | Ref | ||||||
| 5–14.9 | 73 | 0.08 (0.08–0.09) | 0.026 | −0.016 to 0.068 | 0.217 | 0.023 | −0.021 to 0.066 | 0.299 | 0.031 | −0.014 to 0.076 | 0.179 |
| 15–29.9 | 53 | 0.08 (0.08–0.09) | 0.007 | −0.041 to 0.055 | 0.774 | 0.007 | −0.043 to 0.057 | 0.783 | 0.024 | −0.031 to 0.079 | 0.387 |
| ≥30 | 61 | 0.08 (0.08–0.09) | 0.027 | −0.018 to 0.073 | 0.241 | 0.022 | −0.027 to 0.071 | 0.371 | 0.036 | −0.015 to 0.087 | 0.168 |
| Women REM‐AHI, events/h | |||||||||||
| 0–4.9 | 71 | 0.08 (0.07–0.09) | Ref | Ref | Ref | ||||||
| 5–14.9 | 40 | 0.09 (0.08–0.09) | 0.042 | −0.023 to 0.107 | 0.207 | 0.044 | −0.022 to 0.110 | 0.187 | 0.058 | −0.009 to 0.125 | 0.088 |
| 15–29.9 | 46 | 0.08 (0.08–0.10) | 0.032 | −0.033 to 0.096 | 0.331 | 0.038 | −0.028 to 0.103 | 0.263 | 0.049 | −0.018 to 0.116 | 0.153 |
| ≥30 | 96 | 0.09 (0.08–0.10) | 0.075 | 0.018 to 0.133 | 0.010 | 0.074 | 0.014 to 0.133 | 0.015 | 0.079 | 0.019 to 0.139 | 0.010 |
AHI, apnea‐hypopnea index; CI, confidence interval; coef., coefficient; IQR, interquartile range; OSA, obstructive sleep apnea; REM, rapid eye movement.
Results from linear regression analysis with log‐transformed intima thickness as dependent variable. Model 1 adjusted for age, body mass index, alcohol, and smoking. Model 2 adjusted for age, body mass index, alcohol, smoking, systolic blood pressure, low‐density lipoprotein, C‐reactive protein, and diabetes.
Trying to isolate the effect of REM‐OSA, the calculations were repeated in a sub‐sample with an AHI of <15 events/h and in those with a non‐REM AHI of <5 events/h. This reduced the participants with severe REM‐OSA to 36 and 17 individuals, respectively. In these calculations, there was no significant association with REM‐OSA.
4. DISCUSSION
The main finding in this study of a community‐based sample of age‐ and BMI‐matched men and women is that there was an association between severe REM‐OSA and signs of atherosclerosis, which was not explained by age, BMI, smoking, or alcohol. More women than men had severe REM‐OSA; moreover, in the analyses stratified by sex, the association between severe REM‐OSA and intima thickness remained significant only in women.
There are only a few other studies that have investigated the association between REM‐OSA and atherosclerotic CVD. We previously reported that in women, severe REM‐OSA was associated with early signs of atherosclerosis 10 years later (Ljunggren et al., 2018). Our results are also in accordance with a study reporting an association between REM‐OSA and arterial stiffness (Lin et al., 2018), and in patients with CVD, an increased risk of a new cardiovascular event has been reported for patients with severe REM‐OSA (Aurora et al., 2018). The association between REM‐OSA and intima thickness was mainly visible for severe REM‐OSA, this is in accordance with our previous findings (Ljunggren et al., 2018) and the work by Aurora et al. (2018).
There was a stronger association between REM‐OSA and intima thickness than OSA assessed by the AHI or ODI. Furthermore, in the fully adjusted model, only the association with REM‐OSA remained significant. The impact of REM‐OSA on atherosclerosis might be explained by negative effects of REM‐sleep fragmentation or by more severe OSA during REM sleep compared to non‐REM sleep, with longer apneas and deeper desaturations. Possible intermediate mechanisms include inflammation, metabolic dysregulation, hypertension, or dyslipidaemia. In a previous study of a sub‐sample from the SHE cohort, we found that women with severe REM‐OSA had altered levels of inflammatory proteins involved in metabolic regulation and in the atherosclerotic process, while the associations were weaker for overall AHI and ODI (Ljunggren et al., 2020). Thus, obstructive events during REM sleep might trigger a stronger inflammatory response than during non‐REM sleep and thereby contribute to the inflammatory process causing atherosclerosis. REM‐OSA is further associated with increased risk of incident and prevalent hypertension (Appleton et al., 2016; Mokhlesi et al., 2014), while no independent alteration in lipid profile has been reported (Bikov et al., 2019; H. Xu et al., 2020), making a pathway involving dyslipidaemia less likely. In an attempt to address the question of possible intermediate mechanisms, a model also adjusting for systolic blood pressure, LDL, CRP, and diabetes (Model 2) was constructed. However, these covariates did not explain the association between REM‐OSA and intima thickness. It is possible that other inflammatory markers than CRP might better reflect the activated inflammatory pathways, and that systolic blood pressure measured in the morning does not properly reflect the effects of non‐dipping blood pressure (Mokhlesi et al., 2015) during the night.
When stratifying by sex, an association was observed between REM‐OSA and arthrosclerosis in women, but not in men. However, there was no significant interaction based on sex. It is possible that this is explained by differences in power, as fewer men had severe REM‐OSA. Another possibility is that REM‐OSA is a more important marker of cardiovascular risk in women. Aurora et al. (2020) also reported sleep‐state‐specific gender differences in the risk of incident CVD in sleep disordered breathing. Women with isolated REM‐OSA at baseline, who progressed to develop OSA during non‐REM sleep 5 years later, had an increased incidence of CVD, while men with REM‐OSA at baseline did not. Several other recent studies also report higher cardiovascular risk in women with OSA than in men (Kendzerska et al., 2020; Roca et al., 2015; Strausz et al., 2018).This is in contrast to earlier studies, including fewer women with severe OSA, reporting an increased risk of CVD in OSA in men, but not in women (Gottlieb et al., 2010).
The main strengths of this study are the large population‐based sample of individuals, the reliable data from PSG and high‐resolution ultrasonography of the carotid artery, as well as detailed information on relevant confounders. However, there are several limitations that need to be acknowledged. In the sub‐analyses, limited to those with an AHI of <15 events/h or a non‐REM AHI of <5 events/h, we could not find a significant association between REM‐OSA and intima thickness. Although that might be due to a lack of power due to the low number of remaining participants, we cannot claim to have shown the effect of isolated REM‐OSA in the absence of OSA during non‐REM sleep. Also, the cross‐sectional design of the study does not allow for conclusions to be drawn on causality. However, the results in this paper are consistent with the previously published results of a sub‐sample of women in the SHE cohort with a longitudinal design, where severe REM‐OSA at baseline was associated with increased intima thickness 10 years later, also in the sub‐sample with isolated REM‐OSA (Ljunggren et al., 2018). The lower number of individuals with severe OSA based on the AHI or ODI than with severe REM‐OSA, after excluding individuals with CPAP‐treatment, is also a limitation. Hence, the lack of association between the AHI and intima thickness should be interpreted with caution. However, the results from previous epidemiological studies on the association between the AHI and intima‐media thickness are conflicting (Gunnarsson et al., 2014; Souza et al., 2021; Wattanakit et al., 2008; Zhao et al., 2019). Finally, the original female study cohort was oversampled by snoring women, while this was not the case for the men in the MUSTACHE cohort. This affects the prevalence of OSA in the cohorts and could explain a lower power to detect an association in the males.
Despite these limitations, our results add to the increasing evidence that other measures than the total AHI need to be taken into account when diagnosing and treating OSA, showing an association between severe REM‐OSA and early signs of atherosclerosis. A protective effect of OSA treatment has only been shown in those with high treatment adherence (Javaheri et al., 2020). To properly treat REM‐OSA, it is important that the treatment covers the early morning hours when much of the REM sleep occurs. This might be especially important to protect women from additional risk for atherosclerosis from residual untreated REM‐OSA, as women have a greater proportion of their OSA in REM sleep.
5. CONCLUSION
Severe REM‐OSA is associated with early signs of atherosclerosis, and the association is not explained by confounding with age, BMI, smoking, or alcohol. More women than men had severe REM‐OSA; when stratified by sex, the association between severe REM‐OSA and intima thickness was seen in women but not in men.
CONFLICT OF INTEREST
None of the authors has any conflict of interest relevant to the present study.
AUTHOR CONTRIBUTIONS
Study design: Mirjam Ljunggren, Tord Naessén, Jenny Theorell‐Haglöw, Karl A. Franklin and Eva Lindberg. Data collection: Eva Lindberg, Tord Naessén, Karl A. Franklin and Jenny Theorell‐Haglöw. Analysis and interpretation: Mirjam Ljunggren and Eva Lindberg. Writing/reviewing of the manuscript: Mirjam Ljunggren, Tord Naessén, Jenny Theorell‐Haglöw, Karl A. Franklin and Eva Lindberg.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGEMENTS
The study was supported financially by the Swedish Research Council (2020‐02192), Swedish Heart Lung Foundation (20190218) and the Women and Health Foundation.
Ljunggren, M. , Naessén, T. , Theorell‐Haglöw, J. , Franklin, K. A. , & Lindberg, E. (2022). Rapid eye movement sleep apnea and carotid intima thickness in men and women: a SHE‐MUSTACHE cohort study. Journal of Sleep Research, 31(5), e13599. 10.1111/jsr.13599
Funding information Swedish Heart Lung Foundation, Grant/Award Number: 20190218; Vetenskapsrådet, Grant/Award Number: 2020‐02192; Women & Health Foundation
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Akhter, T. , Wikstrom, A. K. , Larsson, M. , & Naessen, T. (2013). Individual common carotid artery wall layer dimensions, but not carotid intima‐media thickness, indicate increased cardiovascular risk in women with preeclampsia: An investigation using noninvasive high‐frequency ultrasound. Circulation. Cardiovascular Imaging, 6(5), 762–768. 10.1161/CIRCIMAGING.113.000295 [DOI] [PubMed] [Google Scholar]
- Appleton, S. L. , Vakulin, A. , Martin, S. A. , Lang, C. J. , Wittert, G. A. , Taylor, A. W. , McEvoy, R. D. , Antic, N. A. , Catcheside, P. G. , & Adams, R. J. (2016). Hypertension is associated with undiagnosed OSA during rapid eye movement sleep. Chest, 150(3), 495–505. 10.1016/j.chest.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Aserinsky, E. , & Kleitman, N. (1953). Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science, 118(3062), 273–274 https://www.ncbi.nlm.nih.gov/pubmed/13089671 [DOI] [PubMed] [Google Scholar]
- Aurora, R. N. , Crainiceanu, C. , Gottlieb, D. J. , Kim, J. S. , & Punjabi, N. M. (2018). Obstructive sleep apnea during REM sleep and cardiovascular disease. American Journal of Respiratory and Critical Care Medicine, 197(5), 653–660. 10.1164/rccm.201706-1112OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora, R. N. , McGuffey, E. J. , & Punjabi, N. M. (2020). Natural history of sleep‐disordered breathing during rapid eye movement sleep. Relevance for incident cardiovascular disease. Annals of the American Thoracic Society, 17(5), 614–620. 10.1513/AnnalsATS.201907-524OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikov, A. , Lazar, Z. , Horvath, P. , Tarnoki, D. L. , Tarnoki, A. D. , Fesus, L. , Horvath, M. , Meszaros, M. , Losonczy, G. , & Kunos, L. (2019). Association between serum lipid profile and obstructive respiratory events during REM and non‐REM sleep. Lung, 197(4), 443–450. 10.1007/s00408-019-00195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. L. , Su, M. C. , Liu, W. H. , Wang, C. C. , Lin, M. C. , & Chen, M. C. (2014). Influence and predicting variables of obstructive sleep apnea on cardiac function and remodeling in patients without congestive heart failure. Journal of Clinical Sleep Medicine, 10(1), 57–64. 10.5664/jcsm.3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert, D. J. , Malhotra, A. , Lo, Y. L. , White, D. P. , & Jordan, A. S. (2009). The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest, 135(4), 957–964. 10.1378/chest.08-2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley, L. J. , Wilhoit, S. C. , & Suratt, P. M. (1985). Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest, 87(4), 432–436 http://www.ncbi.nlm.nih.gov/pubmed/3979129 [DOI] [PubMed] [Google Scholar]
- Gottlieb, D. J. , Yenokyan, G. , Newman, A. B. , O'Connor, G. T. , Punjabi, N. M. , Quan, S. F. , Redline, S. , Resnick, H. E. , Tong, E. K. , Diener‐West, M. , & Shahar, E. (2010). Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation, 122(4), 352–360. 10.1161/CIRCULATIONAHA.109.901801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson, S. I. , Peppard, P. E. , Korcarz, C. E. , Barnet, J. H. , Aeschlimann, S. E. , Hagen, E. W. , Young, T. , Hla, K. M. , & Stein, J. H. (2014). Obstructive sleep apnea is associated with future subclinical carotid artery disease: Thirteen‐year follow‐up from the Wisconsin sleep cohort. Arteriosclerosis, Thrombosis, and Vascular Biology, 34(10), 2338–2342. 10.1161/atvbaha.114.303965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri, S. , Martinez‐Garcia, M. A. , Campos‐Rodriguez, F. , Muriel, A. , & Peker, Y. (2020). Continuous positive airway pressure adherence for prevention of major adverse cerebrovascular and cardiovascular events in obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine, 201(5), 607–610. 10.1164/rccm.201908-1593LE [DOI] [PubMed] [Google Scholar]
- Kendzerska, T. , Leung, R. S. , Atzema, C. L. , Chandy, G. , Meteb, M. , Malhotra, A. , Hawker, G. A. , & Gershon, A. S. (2020). Cardiovascular consequences of obstructive sleep apnea in women: A historical cohort study. Sleep Medicine, 68, 71–79. 10.1016/j.sleep.2019.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. Y. , Ho, C. S. , Tsai, W. C. , & Chen, J. Y. (2018). Different effects of apnea during rapid eye movement period on peripheral arterial stiffness in obstructive sleep apnea. Atherosclerosis, 269, 166–171. 10.1016/j.atherosclerosis.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Lind, L. , Elmstahl, S. , Bergman, E. , Englund, M. , Lindberg, E. , Michaelsson, K. , Nilsson, P. M. , & Sundstrom, J. (2013). EpiHealth: A large population‐based cohort study for investigation of gene‐lifestyle interactions in the pathogenesis of common diseases. European Journal of Epidemiology, 28(2), 189–197. 10.1007/s10654-013-9787-x [DOI] [PubMed] [Google Scholar]
- Ljunggren, M. , Lindahl, B. , Theorell‐Haglow, J. , & Lindberg, E. (2012). Association between obstructive sleep apnea and elevated levels of type B natriuretic peptide in a community‐based sample of women. Sleep, 35(11), 1521–1527. 10.5665/sleep.2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren, M. , Lindberg, E. , Franklin, K. A. , Ohagen, P. , Larsson, M. , Theorell‐Haglow, J. , & Naessen, T. (2018). Obstructive sleep apnea during rapid eye movement sleep is associated with early signs of atherosclerosis in women. Sleep, 41(7), zsy099. 10.1093/sleep/zsy099 [DOI] [PubMed] [Google Scholar]
- Ljunggren, M. , Theorell‐Haglow, J. , Freyhult, E. , Sahlin, C. , Franklin, K. A. , Malinovschi, A. , & Lindberg, E. (2020). Association between proteomics and obstructive sleep apnea phenotypes in a community‐based cohort of women. Journal of Sleep Research, 29(4), e13041. 10.1111/jsr.13041 [DOI] [PubMed] [Google Scholar]
- Marin, J. M. , Carrizo, S. J. , Vicente, E. , & Agusti, A. G. (2005). Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet, 365(9464), 1046–1053. 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- McSharry, D. G. , Saboisky, J. P. , Deyoung, P. , Jordan, A. S. , Trinder, J. , Smales, E. , Hess, L. , Chamberlin, N. L. , & Malhotra, A. (2014). Physiological mechanisms of upper airway hypotonia during REM sleep. Sleep, 37(3), 561–569. 10.5665/sleep.3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhlesi, B. , Finn, L. A. , Hagen, E. W. , Young, T. , Hla, K. M. , Van Cauter, E. , & Peppard, P. E. (2014). Obstructive sleep apnea during REM sleep and hypertension. Results of the Wisconsin sleep cohort. American Journal of Respiratory and Critical Care Medicine, 190(10), 1158–1167. 10.1164/rccm.201406-1136OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhlesi, B. , Hagen, E. W. , Finn, L. A. , Hla, K. M. , Carter, J. R. , & Peppard, P. E. (2015). Obstructive sleep apnoea during REM sleep and incident non‐dipping of nocturnal blood pressure: A longitudinal analysis of the Wisconsin sleep cohort. Thorax, 70(11), 1062–1069. 10.1136/thoraxjnl-2015-207231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, C. , Thornley, K. S. , & Hanly, P. J. (2000). Gender differences in the polysomnographic features of obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine, 161(5), 1465–1472. 10.1164/ajrccm.161.5.9904121 [DOI] [PubMed] [Google Scholar]
- Redline, S. , Yenokyan, G. , Gottlieb, D. J. , Shahar, E. , O'Connor, G. T. , Resnick, H. E. , Diener‐West, M. , Sanders, M. H. , Wolf, P. A. , Geraghty, E. M. , Ali, T. , Lebowitz, M. , & Punjabi, N. M. (2010). Obstructive sleep apnea‐hypopnea and incident stroke: The sleep heart health study. American Journal of Respiratory and Critical Care Medicine, 182(2), 269–277. 10.1164/rccm.200911-1746OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca, G. Q. , Redline, S. , Claggett, B. , Bello, N. , Ballantyne, C. M. , Solomon, S. D. , & Shah, A. M. (2015). Sex‐specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community‐dwelling cohort: The atherosclerosis risk in communities‐sleep heart health study. Circulation, 132(14), 1329–1337. 10.1161/circulationaha.115.016985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Macias, K. A. , Lind, L. , & Naessen, T. (2006). Thicker carotid intima layer and thinner media layer in subjects with cardiovascular diseases. An investigation using noninvasive high‐frequency ultrasound. Atherosclerosis, 189(2), 393–400. 10.1016/j.atherosclerosis.2006.02.020 [DOI] [PubMed] [Google Scholar]
- Sahlin, C. , Franklin, K. A. , Stenlund, H. , & Lindberg, E. (2009). Sleep in women: Normal values for sleep stages and position and the effect of age, obesity, sleep apnea, smoking, alcohol and hypertension. Sleep Medicine, 10(9), 1025–1030. 10.1016/j.sleep.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Somers, V. K. , Dyken, M. E. , Mark, A. L. , & Abboud, F. M. (1993). Sympathetic‐nerve activity during sleep in normal subjects. The New England Journal of Medicine, 328(5), 303–307. 10.1056/NEJM199302043280502 [DOI] [PubMed] [Google Scholar]
- Souza, S. P. , Santos, R. B. , Santos, I. S. , Parise, B. K. , Giatti, S. , Aielo, A. N. , Cunha, L. F. , Silva, W. A. , Bortolotto, L. A. , Lorenzi‐Filho, G. , Lotufo, P. A. , Bensenor, I. M. , & Drager, L. F. (2021). Obstructive sleep apnea, sleep duration, and associated mediators with carotid intima‐media thickness: The ELSA‐Brasil study. Arteriosclerosis, Thrombosis, and Vascular Biology, 41(4), 1549–1557. 10.1161/ATVBAHA.120.315644 [DOI] [PubMed] [Google Scholar]
- Strausz, S. , Havulinna, A. S. , Tuomi, T. , Bachour, A. , Groop, L. , Makitie, A. , Koskinen, S. , Salomaa, V. , & Palotie, T. (2018). Obstructive sleep apnoea and the risk for coronary heart disease and type 2 diabetes: A longitudinal population‐based study in Finland. BMJ Open, 8(10), e022752. 10.1136/bmjopen-2018-022752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanakit, K. , Boland, L. , Punjabi, N. M. , & Shahar, E. (2008). Relation of sleep‐disordered breathing to carotid plaque and intima‐media thickness. Atherosclerosis, 197(1), 125–131. 10.1016/j.atherosclerosis.2007.02.029 [DOI] [PubMed] [Google Scholar]
- Won, C. H. J. , Reid, M. , Sofer, T. , Azarbarzin, A. , Purcell, S. , White, D. , Wellman, A. , Sands, S. , & Redline, S. (2020). Sex differences in obstructive sleep apnea phenotypes, the multi‐ethnic study of atherosclerosis. Sleep, 43(5), zsz274. 10.1093/sleep/zsz274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H. , Xia, Y. , Li, X. , Qian, Y. , Zou, J. , Fang, F. , Yi, H. , Wu, H. , Guan, J. , & Yin, S. (2020). Association between obstructive sleep apnea and lipid metabolism during REM and NREM sleep. Journal of Clinical Sleep Medicine, 16(4), 475–482. 10.5664/jcsm.8242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. , Jin, S. , Li, F. , Jia, G. , Zhang, C. , Zhang, M. , & Zhang, Y. (2021). The diagnostic value of radial and carotid intima thickness measured by high‐resolution ultrasound for ischemic stroke. Journal of the American Society of Echocardiography, 34(1), 72–82. 10.1016/j.echo.2020.09.006 [DOI] [PubMed] [Google Scholar]
- Zhao, Y. Y. , Javaheri, S. , Wang, R. , Guo, N. , Koo, B. B. , Stein, J. H. , Korcarz, C. E. , & Redline, S. (2019). Associations between sleep apnea and subclinical carotid atherosclerosis: The multi‐ethnic study of atherosclerosis. Stroke, 50(12), 3340–3346. 10.1161/STROKEAHA.118.022184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy or ethical restrictions.


