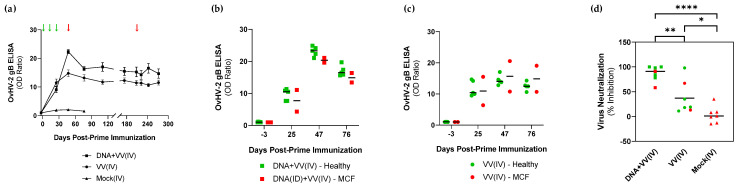
Figure 3.
Anti-OvHV-2 gB antibody response in plasma of animals immunized with OvHV-2 DNA plus AlHV-1-vectored vaccine [DNA+VV(IV)], AlHV-1-vectored vaccine only [VV(IV)], or mock control [Mock(IV)] and challenged with OvHV-2 at 49 days post-prime immunization in Experiment 1. (a) Anti-OvHV-2 gB antibody responses pre- and post-immunizations and challenge measured by ELISA; error bars represent SEM and arrows represent immunizations (green) and challenges (red) times. (b,c) Individual anti-OvHV-2 gB antibody response in animals that remained healthy or developed SA-MCF following OvHV-2 challenge in the DNA+VV(IV) (b) and VV(IV) (c) groups; bars indicate the median. (d) Individual anti-OvHV-2 gB neutralizing antibody response measured by viral neutralization assay in each group at 46 days post-prime immunization; green symbols refer to animals that remained healthy after challenge and red symbols to animals that developed MCF; bars indicate mean; **** p ≤ 0.0001, ** p = 0.0022, and * p = 0.