Abstract
Introduction
To date, no anti‐reflux operations have been reported with the new da Vinci Single‐Port (single port (SP)) robotic platform. We aimed to describe this novel surgical approach and evaluate its safety and feasibility.
Methods
All robotic SP operations were performed under an Institutional Review Board approved protocol.
Results
Two patients underwent robotic SP anti‐reflux surgery through a single incision of 2.7 cm (one Nissen‐fundoplication and one re‐Redo Nissen‐fundoplication). The mean docking‐time was 2.5 (2–3) minutes and mean console‐time was 147 (119–155) minutes. No additional ports were needed, and no intraoperative complications occurred. Patients tolerated a soft diet on postoperative day 1 and were discharged on POD‐2 and 3.
Conclusion
Robotic SP anti‐reflux surgery appears to be safe and feasible. This platform offers similar advantages to the multiport robotic surgery, while adding lower invasiveness and an improved cosmesis. Further studies are needed to confirm our results and evaluate long‐term outcomes of this surgical approach.
Keywords: da Vinci single port (SP), gastro‐oesophageal reflux disease (GERD), minimally invasive surgery (MIS), single port robotic surgery
1. INTRODUCTION
In the last decades, minimally invasive surgery has been widely adopted for the surgical treatment of gastroesophageal reflux disease (Gastro‐oesophageal reflux disease (GERD)) due to its proven benefits, as compared to the open approach. 1 , 2 , 3 , 4 Although laparoscopy remains the most commonly used surgical approach, robotic surgery has proven to be a safe minimally invasive approach with potential surgical advantages and excellent functional outcomes. 5
To further minimise the invasiveness of multiport surgery, single incision procedures were developed. However, single incision laparoscopic surgery (SILS) and the robotic single site technique never reached widespread acceptance due to a significant loss of ergonomics, high learning curve, limited assistance, and counterintuitive instruments. 6 , 7 , 8
However, a new robotic single port (SP) platform that might overcome previous single‐site surgery drawbacks has been recently developed. This system has three multi‐jointed wristed instruments and a three‐dimensional high definition (3D‐HD) articulating scope, which offers ideal instrument triangulation (Figure 1).
FIGURE 1.

Multi‐jointed wristed instruments and 3D‐HD articulating scope
As the da Vinci SP platform is not yet approved by the U.S. Food and Drug Administration for general surgery operations, all procedures performed with this platform are under an Institutional Review Board (IRB) approved protocol. In addition, all patients sign a consent form that details the experimental nature of this approach.
We aimed to describe the technique of robotic SP anti‐reflux surgery and evaluate its safety and feasibility.
2. METHODS
All robotic SP operations were performed by a single surgeon (Francesco M. Bianco) under the IRB protocol #2021‐0520. Herein we present the initial experience, at our institution, using the robotic SP platform for patients with GERD.
3. ROBOTIC SYSTEM
The da Vinci SP system is composed by a surgeon console, a patient side cart, and a vision cart. The system utilises a similar surgeon console as the other Intuitive surgical platforms. The main difference is the presence of an instruments' navigation system that shows the real‐time position of robotic instruments and camera. The vision cart contains the optic, light source, energy, and camera attachment maintaining the same functions and interactions as previous systems. The patient side cart has a completely redesigned structure with a single arm architecture that hosts three multi jointed, wristed instruments and a 3D HD articulating scope (Figure 2). Moreover, the improved functions of this optic allow an easily conversion from upside to downside view that can facilitate the dissection in narrow access areas.
FIGURE 2.

da Vinci single port (SP) patient side cart arm
4. SURGICAL TECHNIQUE
After induction of general anaesthesia and endotracheal intubation, the patient is placed supine on a bean‐bag with parted legs and a 20° reverse Trendelenburg. The procedure starts by creation of a single access in the left upper quadrant through a 2.7 cm incision.
The SP robot is then positioned at the patient's right side and docked to the SP trocar. Through this SP, the robotic camera, a Cadière forceps, a fenestrated bipolar forceps and a cautery hook are progressively inserted (Figure 3). The scope is placed cranially in the oval cannula lumen. This helps to achieve a clear visualisation of the hiatus and gastroesophageal junction (GEJ). The instruments are then inserted through the two lateral and the lower circular lumens (Figure 4). The instruments and camera need to be inserted 10 cm into the body before their elbows can clear the tip of the metal cannula. Therefore, to reach the ideal instrument separation and triangulation to operate, the port should be at least 10 cm from the closest edge and at a maximum 25 cm from the furthest edge of the surgical field (Figure 5).
FIGURE 3.
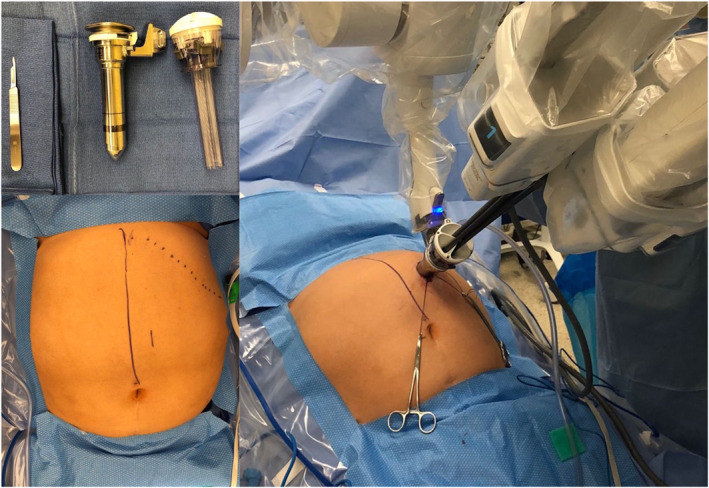
Port placement of da Vinci single port (SP) system for anti‐reflux surgery
FIGURE 4.
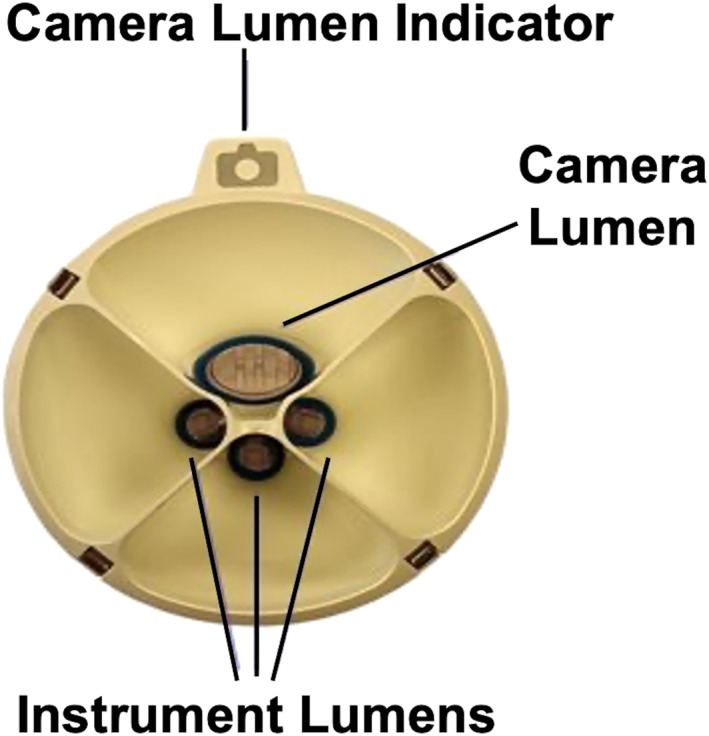
Instrument and scope positioning
FIGURE 5.
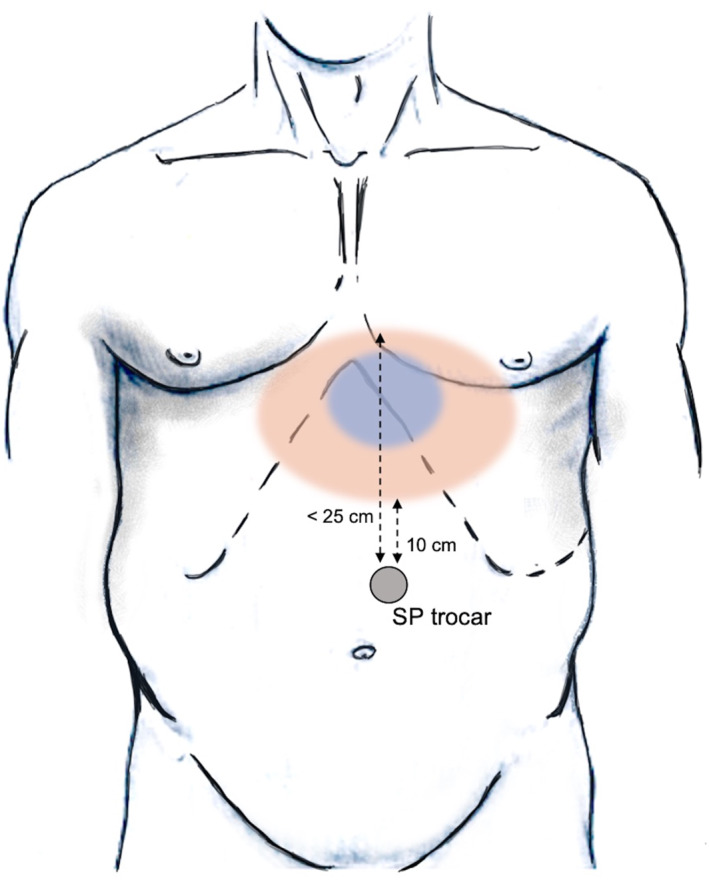
Surgical workspace and minimum/maximum distances
4.1. Single‐port robot‐assisted Nissen fundoplication (Video 1 ‐ https://youtu.be/ANTjkQYAiZE)
The left triangular hepatic ligament is transected and used as anchoring point to the falciform ligament using a temporary V‐lock suture (Figure 6). This manoeuver allows to retract the left hepatic lobe to achieve an adequate exposure of the surgical field.
FIGURE 6.
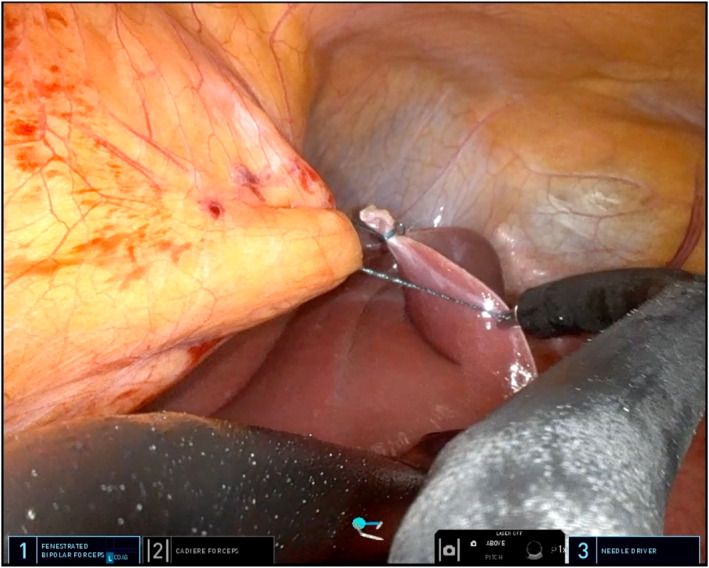
Anchoring of the left triangular hepatic ligament
The stomach is reduced into the abdominal cavity with the Cadiere forceps introduced through the lower circular cannula lumen. The GEJ is carefully dissected and the hernia sac is progressively detached from the diaphragmatic pillars with the monopolar hook and the bipolar forceps inserted through the left and right cannula lumens.
A small retroesophageal window is created and a penrose drain is placed (Figure 7A–B). During the posterior oesophageal dissection, the stomach is lifted using one of the lateral instruments to achieve adequate exposure. The retroesophageal dissection is then carried out using the lower and the contralateral robotic instruments (Figure 8).
FIGURE 7.
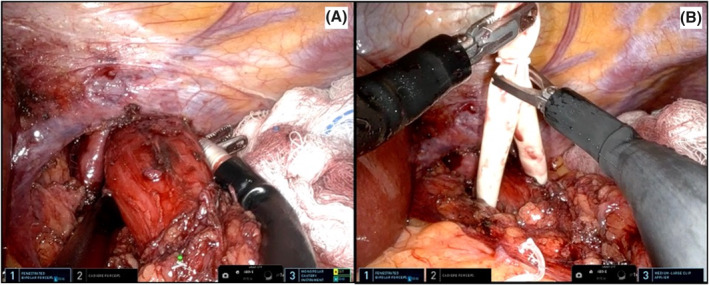
A–B Preparation of a retroesophageal window and penrose drain placement
FIGURE 8.
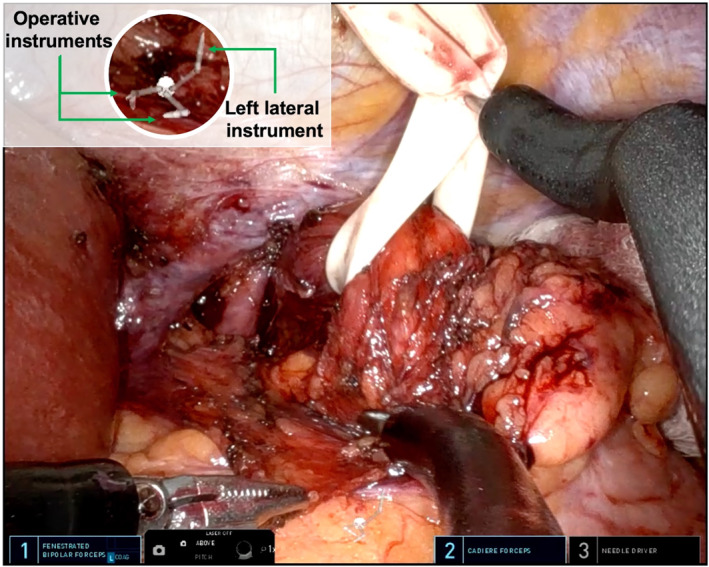
Upward gastroesophageal junction (GEJ) retraction during the retroesophageal dissection
The penrose drain is retracted downwards to complete the mediastinal mobilisation of the oesophagus for about 7 cm, obtaining at least 3 cm of distal oesophagus into the abdominal cavity. The mobilisation of the gastric fundus is achieved identifying and preserving the short gastric vessels. Once the dissection is completed, the crural closure is performed posteriorly to the oesophagus using interrupted permanent sutures. A Nissen fundoplication is constructed passing the gastric fundus posteriorly to the oesophagus in a standard fashion (Figure 9A–B). A 50 French bougie is used to calibrate the wrap which is then constructed with three interrupted prolene stitches.
FIGURE 9.
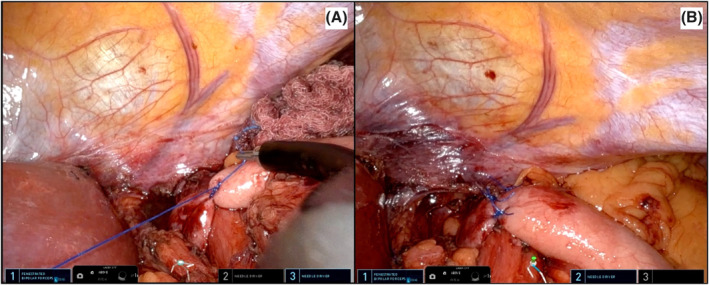
A–B Construction of the Nissen fundoplication
4.2. Single‐port robot‐assisted re‐Redo Nissen fundoplication (Video 2 ‐ https://youtu.be/9X6rYPVtmJk)
In case of redo procedure, the transection of the left triangular hepatic ligament and an extended adhesiolysis may be required to access the surgical target. These manoeuvres are performed using a combination of blunt and sharp dissection. After the identification of the right diaphragmatic crus, the distal oesophagus is carefully dissected. When the surgical indication is a malfunctioning of the fundoplication and the GEJ is already localised in the abdomen, the dissection can be performed even only anteriorly in order to carry out the recalibration of the wraps without compromising the stability of the GEJ. In that case, the hiatoplasty is realized anteriorly to the oesophagus using interrupted permanent sutures. Once completed the identification of the old fundoplication wrap, the recalibration is performed with three interrupted non‐absorbable sutures.
5. RESULTS
Patient characteristics are summarised in Table 1. Both operations were completed successfully without intraoperative complications, need for additional transabdominal ports or conversion. Estimated blood loss was 10 ml in both cases. Each procedure was completed with one robotic docking and the mean time to complete this phase was 2 min. Operative characteristics, docking and console times are reported in Table 2. Both patients were advanced to a soft diet on postoperative day (POD) 1, after the realization of a control upper‐GI study.
TABLE 1.
Patient characteristics
| Case | Age | Gender | BMI | ASA | Diagnosis | Previous abdominal surgery |
|---|---|---|---|---|---|---|
| 1 | 60 | F | 31 | 3 | GERD not responding to PPIs with a moderate‐sized sliding hiatal hernia | Yes |
| 2 | 68 | F | 26.5 | 3 | Loose Nissen wrap with Grade B oesophagitis | Yes |
Abbreviaitons: ASA, American Society of Anaesthesiologists; BMI, body mass index.
TABLE 2.
Operative characteristics
| Case | EBL (cc) | Total operative time (min) | Number of dockings | Docking time (min) | Console time (min) |
|---|---|---|---|---|---|
| 1 | 10 | 139 | 1 | 3 | 136 |
| 2 | 10 | 155 | 1 | 2 | 153 |
Abbreviaiton: EBL, estimated blood loss.
With an uneventful postoperative course, the re‐Redo procedure was discharged on POD 2 and the primary Nissen procedure on POD 3. At the subsequent follow‐up visits both patients denied dysphagia and reported a complete resolution of reflux symptoms after the procedure.
6. DISCUSSION
The robotic platform provides surgeons with a 3‐D, 10‐fold magnified view of the operating field, restores the natural hand–eye coordination axis, and through its articulating surgical instruments offers a high degree of freedom. The result of this technological improvement is a more intuitive and precise dissection in a confined surgical field with impressive dexterity. 9 , 10 A recent investigation showed that between 2012 and 2018, the use of the robotic platform to perform antireflux procedures increased from 5.4% to 26%. 11
Despite this widespread diffusion, several randomized control trials and observational studies failed to prove the superiority of the robotic approach to laparoscopy in antireflux procedures. 12 , 13 , 14
However, a recent analysis on 293 elective hiatal hernia procedures performed laparoscopically (n = 151) and robotically (n = 142) showed a reduced length of hospital stay (1.3 ± 1.8 vs. 1.8 ± 1.5, p = 0.003) and postoperative complications (6.3 vs. 19.2%, p = 0.001) with the robotic approach. 15
In our experience, the robotic SP platform provides the benefits of multiport robotic surgery, while adding a lower invasiveness and an improved cosmesis. Interestingly, previous surgical approaches using SP technique have been used without achieving popularity in the surgical community. For instance, since the first SILS‐Nissen fundoplication procedures reported in 2010 by Hamzaoglu et al., 16 only few other series have been described in literature with this approach. Although some comparative studies reported encouraging results with similar outcomes to the conventional multiport approach, the SILS antireflux procedure showed longer learning curve and operative times. 6 , 7 , 8 The main difficulties encountered with this approach were reduced ability to triangulate, instrument collision, poor ergonomics, and a two‐dimensional view.
Those limitations were only partially reduced with the subsequent introduction of the robotic single‐site approach. The attempt to reproduce the less invasive SILS, was first described in 2009 by Kaouk et al. who first reported a small series of robotic single‐site urological procedures with promising results. 17 Despite different procedures have already been described, the only report of Nissen fundoplication with this novel approach was performed on a nonsurvival porcine model in 2010. 18 However, when compared to the multiport robot, this single‐site approach still presented lack of endowrist motility, which significantly limited its widespread acceptance.
In 2018, the launch of the new da Vinci SP surgical system represented a substantial improvement in the mini‐invasive approach with single incision technique. This new technology was designed to apply the powerful capabilities of the multiport robotic platform to single‐port surgery with an ideal distal instrument triangulation. The multi‐jointed, fully wristed, elbowed instruments eliminated the instruments clashing affecting the laparoscopic and robotic single site approaches.
The instruments are introduced through a 25 mm diameter port and are properly triangulated around the target anatomy. Moreover, these manoeuvres are facilitated by the 360‐degree rotating boom‐mounted arm of the SP cart which allows an easy access to different surgical targets during the same procedure. However, the absence of a dedicated vessel sealer, stapler, and suction devices may be considered as a significant drawback of the SP system.
We used an IRB approved protocol to start our experience with the new SP platform at the University of Illinois at Chicago (USA). To date, more than 275 robotic SP procedures have been performed by a single surgeon (Francesco M. Bianco) with an extensive experience in single site laparoscopic and robotic surgery. 19 To our knowledge, we have described the first robotic SP anti‐reflux procedures in the literature. In our initial experience, this novel approach has been shown to be safe and feasible, even for a re‐Redo procedure which is known to be significantly more challenging. However, in patients with giant hiatal hernias, the standard multiport approach is preferable due to the wider instruments divergent action and stronger traction and countertraction manoeuvres that allows an easier hernia dissection and reduction. Moreover, a common concern following single incision procedures, which require a larger fascial incision, is the risk of incisional hernias. Long term follow‐up studies are required to determine if this approach is associated with a high incisional hernia rates. Larger series on SP anti‐reflux procedures are needed to confirm our results before recommending this approach for the surgical treatment of GERD.
7. CONCLUSION
Robotic SP anti‐reflux surgery seems to be safe and feasible. This platform offers similar advantages to the multiport robotic surgery, while adding lower invasiveness and an improved cosmesis. Further studies are needed to confirm our results and evaluate long‐term outcomes of this surgical approach.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest or financial ties to disclose.
Drs. Antonio Cubisino; Nicolas H. Dreifuss; Francisco Schlottmann; Carolina Baz; Alberto Mangano; Mario A. Masrur and Francesco M. Bianco have no conflicts of interest or financial ties to disclose.
Supporting information
Video S1
Video S2
ACKNOWLEDGEMENTS
The authors would like to thank Ashley Farinelli for the voice contribution to the videos herein presented. The authors received no specific funding for this work.
Cubisino A, Dreifuss NH, Schlottmann F, et al. Robotic single port anti‐reflux surgery: Initial worldwide experience of two cases with a novel surgical approach to treat gastroesophageal reflux disease. Int J Med Robot. 2022;18(6):e2437. 10.1002/rcs.2437
DATA AVAILABILITY STATEMENT
Data available in article supplementary material.
REFERENCES
- 1. Stefanidis D, Hope WW, Reardon PR, Richardson WS, Fanelli RD, SAGES Guidelines Committee . Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24(11):2647‐2669. 10.1007/s00464-010-1267-8 [DOI] [PubMed] [Google Scholar]
- 2. Peters MJ, Mukhtar A, Yunus RM, et al. Meta‐analysis of randomized Clinical trials comparing open and laparoscopic anti‐reflux surgery. Am J Gastroenterol. 2009;104(6):1548‐1561. 10.1038/ajg.2009.176 [DOI] [PubMed] [Google Scholar]
- 3. Laine S, Rantala A, Gullichsen R, Ovaska J. Laparoscopic vs conventional Nissen fundoplication: a prospective randomized study. Surg Endosc. 1997;11(5):441‐444. 10.1007/s004649900386 [DOI] [PubMed] [Google Scholar]
- 4. Schlottmann F, Strassle PD, Patti MG. Comparative analysis of perioperative outcomes and Costs between laparoscopic and open antireflux surgery. J Am Coll Surg. 2017;224(3):327‐333. 10.1016/j.jamcollsurg.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 5. Elmously A, Gray KD, Ullmann TM, Fahey TJ, Afaneh C, Zarnegar R. Robotic reoperative anti‐reflux surgery: low perioperative morbidity and high symptom resolution. World J Surg. 2018;42(12):4014‐4021. 10.1007/s00268-018-4708-5 [DOI] [PubMed] [Google Scholar]
- 6. Fukuda S, Nakajima K, Miyazaki Y, et al. Laparoscopic surgery for esophageal achalasia: multiport vs single‐incision approach: laparoscopic achalasia: multi vs single. Asian J Endosc Surg. 2016;9(1):14‐20. 10.1111/ases.12226 [DOI] [PubMed] [Google Scholar]
- 7. Sharp NE, Vassaur J, Buckley FP. Single‐site nissen fundoplication versus laparoscopic nissen fundoplication. J Soc Laparoendosc Surg. 2014;18(3):e2014.00202. 10.4293/JSLS.2014.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perivoliotis K, Sarakatsianou C, Tepetes K, Baloyiannis I. Single incision laparoscopic fundoplication: a systematic review of the literature. WJGS. 2019;11(3):179‐190. 10.4240/wjgs.v11.i3.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kenngott HG, Fischer L, Nickel F, Rom J, Rassweiler J, Müller‐Stich BP. Status of robotic assistance—a less traumatic and more accurate minimally invasive surgery? Langenbecks Arch Surg. 2012;397(3):333‐341. 10.1007/s00423-011-0859-7 [DOI] [PubMed] [Google Scholar]
- 10. Suda K, Nakauchi M, Inaba K, Ishida Y, Uyama I. Robotic surgery for upper gastrointestinal cancer: Current status and future perspectives: robotic upper GI surgery. Dig Endosc. 2016;28(7):701‐713. 10.1111/den.12697 [DOI] [PubMed] [Google Scholar]
- 11. Sheetz KH, Claflin J, Dimick JB. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open. 2020;3(1):e1918911. 10.1001/jamanetworkopen.2019.18911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Müller‐Stich BP, Reiter MA, Wente MN, et al. Robot‐assisted versus conventional laparoscopic fundoplication: short‐term outcome of a pilot randomized controlled trial. Surg Endosc. 2007;21(10):1800‐1805. 10.1007/s00464-007-9268-y [DOI] [PubMed] [Google Scholar]
- 13. Müller‐Stich BP, Reiter MA, Mehrabi A, et al. No relevant difference in quality of life and functional outcome at 12 months’ follow‐up—a randomised controlled trial comparing robot‐assisted versus conventional laparoscopic Nissen fundoplication. Langenbecks Arch Surg. 2009;394(3):441‐446. 10.1007/s00423-008-0446-8 [DOI] [PubMed] [Google Scholar]
- 14. Lang F, Huber A, Kowalewski KF, et al. Randomized controlled trial of robotic‐assisted versus conventional laparoscopic fundoplication: 12 years follow‐up. Surg Endosc. 2022;25. Published online. 10.1007/s00464-021-08969-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soliman BG, Nguyen DT, Chan EY, et al. Robot‐assisted hiatal hernia repair demonstrates favorable short‐term outcomes compared to laparoscopic hiatal hernia repair. Surg Endosc. 2020;34(6):2495‐2502. 10.1007/s00464-019-07055-8 [DOI] [PubMed] [Google Scholar]
- 16. Hamzaoglu I, Karahasanoglu T, Aytac E, Karatas A, Baca B. Transumbilical totally laparoscopic single‐port nissen fundoplication: a new method of liver retraction: the Istanbul technique. J Gastrointest Surg. 2010;14(6):1035‐1039. 10.1007/s11605-010-1183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaouk JH, Goel RK, Haber GP, Crouzet S, Stein RJ. Robotic single‐port transumbilical surgery in humans: initial report. BJU Int. 2009;103(3):366‐369. 10.1111/j.1464-410X.2008.07949.x [DOI] [PubMed] [Google Scholar]
- 18. Allemann P. Robotics may overcome technical limitations of single‐trocar surgery: an experimental prospective study of nissen fundoplication. Arch Surg. 2010;145(3):267. 10.1001/archsurg.2009.295 [DOI] [PubMed] [Google Scholar]
- 19. Dreifuss NH, Schlottmann F, Cubisino A, Bianco FM. Novel surgical approach for gastric gastrointestinal stromal tumor (GIST): robotic single port partial gastrectomy. Surg Oncol. 2022;40:101704. 10.1016/j.suronc.2021.101704 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1
Video S2
Data Availability Statement
Data available in article supplementary material.


