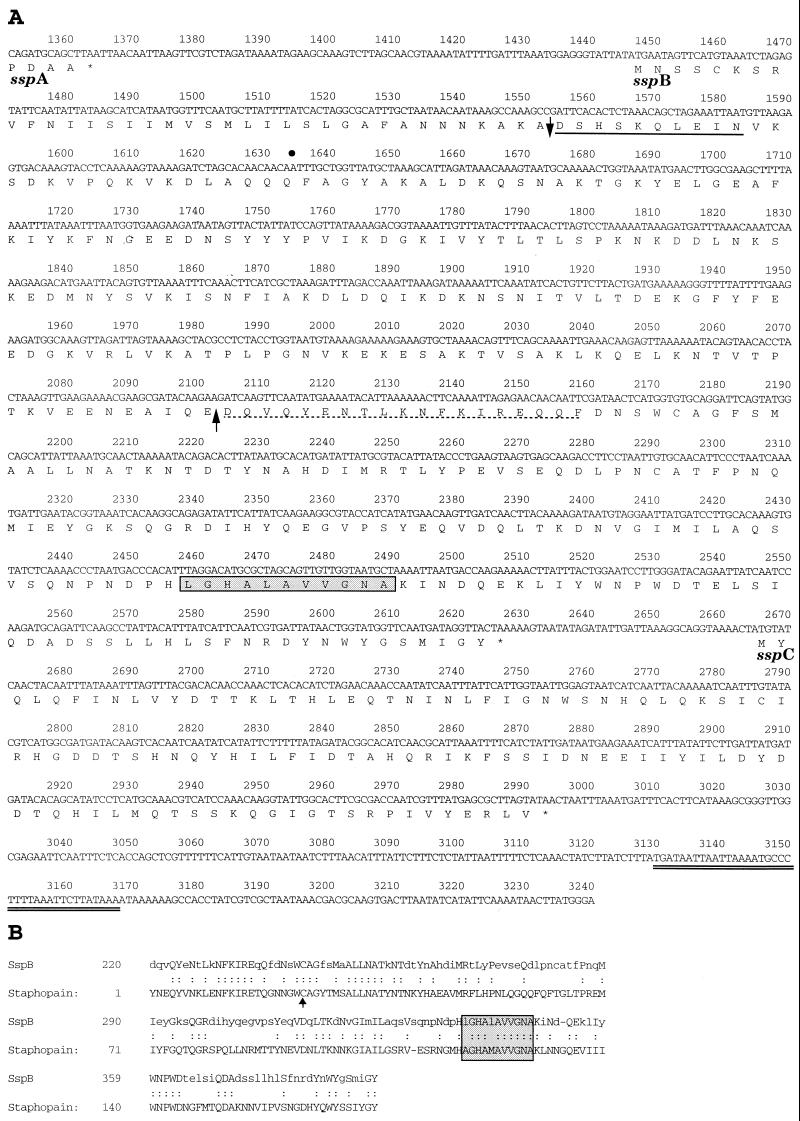
FIG. 1.
Nucleotide and deduced amino acid sequence of the sspB and sspC genes from S. aureus strain COL (A) and alignment of the C-terminal region of SspB with the staphopain cysteine protease (23) purified from S. aureus strain V8 (B). (A) The sspA open reading frame encoding V8 protease terminates at nucleotide 1364, and the nucleotide coordinates of the ssp operon are additive to the original ssp sequence of S. aureus strain V8 (9). The 3′ end of the published ssp sequence terminates at nucleotide 1634 of the ssp operon, indicated by a bullet. Stop codons are indicated by asterisks. The predicted signal peptide cleavage site in the SspB amino acid sequence is indicated by a downward-pointing arrow, and the experimentally determined N-terminal sequence obtained from the 41-kDa SspB protein secreted by S. aureus SP6391 is represented by a solid underbar. The dashed underbar represents the N-terminal sequence of a 22-kDa protein secreted in increased abundance in a sarA mutant of S. aureus 8325-4 (10). An upward-pointing arrow indicates a potential processing site for cleavage of SspB at Glu219 by SspA to form a mature cysteine protease. The double underbar following the sspC open reading frame indicates a hairpin structure, representing a putative transcriptional termination signal. (B) The arrow points to an active site cysteine residue in staphopain, which has been determined by crystallography to bind the cysteine protease inhibitor, E-64 (23). The boxed and shaded residues in both panels represent a pattern of amino acids that is identified as a eukaryotic thiol protease histidine active site. Identical amino acids are indicated by colons, and lowercase letters represent nonidentical amino acids.