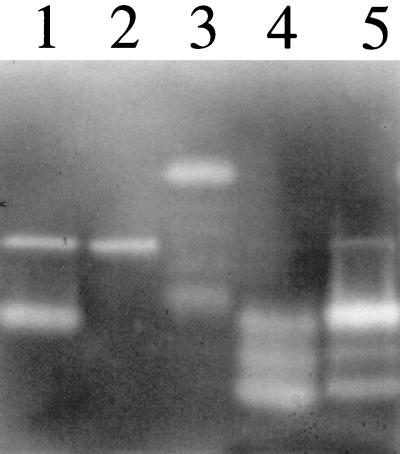
FIG. 5.
Zymogram analysis demonstrating conversion of SspB into lower-molecular-mass forms by treatment with purified SspA. The assay was performed as described above for Fig. 4B. Lane 1, 150 ng of V8 protease in PBS containing 1 mM cysteine; lane 2, 150 ng V8 protease pretreated with 10 mM DFP serine protease inhibitor; lane 3, 0.025 OD600 units of stationary-phase culture supernatant from SP6912; lane 4, supernatant from 0.08 OD600 units of SP6912 culture, incubated for 60 min in PBS containing 300 ng of purified V8 protease and 1 mM cysteine, followed by addition of 10 mM DFP; lane 5, identical to lane 4, except that DFP treatment was omitted. The higher-molecular-mass activity in lanes 1 and 2 containing purified SspA represents a shift in mobility that is observed when the sample is incubated in a reducing agent prior to electrophoresis. Coomassie blue staining of SDS-PAGE gels containing reduced or nonreduced SspA has confirmed that SspA exhibits faster migration when nonreduced (data not shown).