Abstract
Background
Cellular metabolism and exposure to solar irradiation result in generation of free radicals which are destructive and can lead to premature aging. Antioxidants and free radical scavengers such as carotenoids successfully protect from these free radicals by quenching and neutralizing them thereby strengthening skin barrier which leads to improved skin moisturization, desquamation, and a more youthful look. This study was designed to evaluate the consumer‐perceived efficacy of an oral supplement (Lumenato™) containing a mix of tomato carotenoids and oil‐soluble vitamins in improving skin appearance after 12 weeks of supplement use.
Materials and Methods
Plasma levels of phytoene, phytofluene, zeta‐carotene, and lycopene were quantitated before and after 1‐, 2‐, 3‐, and 4‐week administration of Lumenato by 24 healthy volunteers. Part II of the study addressed skin visual attributes as assessed by validated tools (questionnaires). A total of 60 females, aged 35 to 55 years, completed part II of the study. The subjects answered questionnaires pertaining to their assessment of skin appearance before and after 12 weeks of taking the supplement.
Results
There was a significant increase (p < 0.001) in plasma levels of phytoene, phytofluene, and zeta‐carotene after 1‐ to 4‐week treatment with Lumenato. After 12 weeks of using the supplement, the score of different skin parameters was reported to significantly improve (p < 0.001). Improvement was recorded in skin elasticity, firmness, brightness, skin tone, reduction in dark spots and periorbital dark circles, skin hydration, texture and fine lines and wrinkles. A significant (p < 0.001) improvement in overall skin condition after using the supplement was observed. The subjects noticed statistically significant (p < 0.001) improvement in skin elasticity, firmness, brightness, skin tone, reduction in dark spots and periorbital dark circles, skin hydration, texture and fine lines and wrinkles after 12 weeks of using the supplement. The overall skin condition also exhibited a significant improvement (p < 0.001). Self‐assessed improvement of the face was identified at the first time point (4 weeks) and improved significantly (p < 0.001) for the 12 weeks of use. Interestingly, these improvements persisted even after treatment was stopped.
Conclusion
Based on the confines and conditions of this study, the use of oral supplement containing a mix of tomato carotenoids significantly increased plasma levels of phytoene, phytofluene, and zeta‐carotene, and continuous use resulted in improved facial skin attributes which were palpable by the consumers and continued even after treatment was stopped.
Keywords: anti aging, carotenes, phytoene, phytofluene, wrinkles
1. INTRODUCTION
Aging and the degenerative diseases associated with it have been declared to be attributed to the deleterious strikes of free radicals on cell constituents and connected tissues. 1 Free radicals are continuously generated in the body as a result of cellular metabolism 2 exposure to irradiation with UV, visible light (VIS), and infrared. 3 , 4 , 5 These extremely reactive molecules can destroy cells and cell compartments, disrupt skin barrier, and display signs of premature aging. 6 , 7
The stratum corneum of the skin at the interface between body and environment is directly exposed to a prooxidative environment, including UV irradiation, chemical oxidants, air pollutants, and microorganisms. 8 It acts as the primary barrier to block penetration of undesirable molecules into the body and water loss from the body. It is comprised of a unique, highly lipophilic 2‐compartment system of structural, enucleated cells (corneocytes) embedded in a lipid‐enriched intercellular matrix, forming stacks of bilayers that are rich in ceramides, cholesterol, and free fatty acids. 9 The stratum corneum lipid composition and structure play a key role in determining barrier integrity, which is essential for skin moisturization, normal desquamation, and healthy, young‐looking skin. 10
Oxygen radicals and other activated oxygen species cause modifications of the amino acids of stratum corneum proteins that result in functional changes in structural or enzymatic proteins. 11 Protein carbonyls may be formed either by oxidative cleavage of proteins or by direct oxidation of lysine, arginine, proline, and threonine residues. In addition, carbonyl groups may be introduced into proteins by reactions with aldehydes (4‐hydroxy‐2‐nonenal, malondialdehyde) produced during lipid peroxidation or with reactive carbonyl derivatives generated as a consequence of the reaction of reducing sugars or their oxidation products with lysine residues of proteins. 12 Increase in carbonyl groups and protein oxidation has been associated with disrupted skin barrier and signs of premature aging. 13 , 14 The protein oxidation can be counteracted by antioxidants.
Increased levels of free radicals can reach a critical level leading to oxidative stress 15 , 16 which can manifest as premature skin aging, 17 , 18 immunosuppression, and even cancer. 19 , 20 Thus, it is extremely important to control and quench these free radical accumulations. Fortunately, the human body possesses an efficient antioxidant network to protect from the destructive effect of these free radicals. 3 , 21 , 22 , 23 Interaction of antioxidants with free radicals gives rise to the destruction of antioxidants and neutralization of free radicals. 24 Many of these antioxidants cannot be synthesized by the human body entirely or in sufficient amounts and must be administered via rich nutrition of phytonutrients. 25 , 26 , 27 , 28
Human skin is equipped with a network of enzymatic and nonenzymatic antioxidant systems to neutralize and combat oxidative insult of the environment. 29 Studies have found a gradient of antioxidants such as vitamin E, glutathione, uric acid, catalase, and ascorbate gradient in the stratum corneum of healthy human skin, with the lowest antioxidant concentrations on the surface and the highest in the deepest stratum corneum layers. 30 , 31 , 32 In addition to protection from lipid peroxidation, antioxidants are reported to stabilize the lipid bilayer, 33 thereby strengthening the skin barrier.
Topically applied 34 and systemically administered antioxidants provide protection from UV‐induced oxidative damage in stratum corneum. 29 Antioxidants such as carotenoids accumulate in the stratum corneum and retard oxidation and cross‐linking of the keratin chains, resulting in a diminution of UVB radiation reaching potential epidermal target sites 29 , 35 thereby protecting the skin tissues including skin barrier.
The interaction of free radicals with antioxidants is of importance in the development of prevention strategies against skin aging. The richly colored carotenoids such as lutein, lycopene, and β carotene have been shown to provide cosmetic improvements to skin when applied topically on skin 36 or used as oral supplements. 37 Carotenoids are able to repulse several attacks of free radicals before being destroyed. 38 , 39 These antioxidant and anti‐inflammatory properties of carotenes provide protection from the damaging ultraviolet irradiation.
Decades of research support indicate that several skin products that include carotenoids are effective in restoring some of the qualities identified with more youthful, healthier skin. It is well accepted that beauty can arise from within the body. Consumers are increasingly comfortable with ingesting nutrients to enhance skin beauty and a healthful appearance. 37 , 40 A variety of treatments with colored carotenoids show that they may be helpful in cosmetic roles to provide some UV protection and reduce oxidation and inflammation damage. Ingestion of colored carotenoids may also produce some darkening or color change in complexion, desired by some consumers.
It is well known that consumption of carotenoid‐rich tomatoes is associated with decreased risks for several chronic diseases, most notably prostate cancer and cardiovascular disease (CVD). 41 , 42 , 43 Lycopene and other colorless carotenoids found in tomatoes, such as phytoene and phytofluene, are postulated to have biological activity. Ingested carotenoids are absorbed into the enterocyte and packaged into chylomicrons which are secreted into the lymph, entering the systemic circulation primarily via the thoracic duct. They are repackaged by hepatic lipoprotein production and shuttled in the plasma to tissues by lipoproteins. 44 Carotenoids from plasma are partitioned into different tissues of the body including the skin.
Carotenoids of human plasma can be increased by moderate alterations in diet within a short time. 45 , 46 Literature indicates substantial interindividual heterogeneity in blood levels of carotenoids 47 ; nevertheless, several studies indicate that ingestion of carotenoid result in their increase in skin stipulating that carotenoid concentration in the skin reflects the lifestyle of individuals. 48 , 49 , 50 A high level of carotenoids can be achieved with a healthy diet rich, for instance, in fruit and vegetables. Stress factors such as illness, UV and IR radiation of the sun, smoking, and alcohol consumption reduce the concentration of the carotenoids in the skin. Meanwhile, premature skin aging is less pronounced in people with high levels of antioxidants in their tissues. 48
Claims associated with the reversal of aging signs such as reduced facial wrinkling are often subjected to intense scrutiny both from the regulators and competitors. Instrumental evaluation is often used in an attempt to provide data to support claims, 51 and a deluge of instruments is available to measure changes in skin that can provide quantitative results. However, this approach has been criticized since the changes measured by instruments can be too small to be perceptible by the consumers. It is thus a good recommendation to meld the instrumental measurement with subject self‐assessments. 52 Furthermore, the final approval of consumers is what signifies the success of a product. Therefore, subject self‐assessment is of the most importance in the determination of the efficacy of a product. This clinical study was designed to study the effect of a supplement on improving skin visual attributes perceived by subject. A study by Tarshish et al. 37 reports the instrumental measurement of changes in skin after using a carotenoid supplement ingestion, while this report only addresses the subject self‐assessment of perceived changes in signs of skin aging after using the supplement for 12 weeks.
2. ACTIVE INGREDIENTS
The capsules being evaluated in this study contain tomato actives such as carotenoids with ability to absorb light in the damaging UV wavelengths and provide anti‐inflammation and anti‐oxidation effects. 40 They are expected to have the potential to restore skin to more youthful structural features and healthier appearance. Other studies with carotenoid‐rich extracts demonstrate that users perceive their skin as healthier and more youthful and beautiful after ingesting tomato‐based supplements. 37 , 53
Colorless carotenoids phytoene and phytofluene are precursors of well‐known and colorful carotenoids. These two molecules are major dietary carotenoids and are present in common foods such as melon, banana, carrot, pepper, avocado, and tomatoes. Normal dietary intakes of phytoene and phytofluene have been shown to be 2.0 and 0.7 mg, respectively, 54 and these carotenoids have been shown to accumulate in human blood and other tissues. In human skin, phytoene and phytofluene accumulate at higher concentrations than lycopene. 53
A variety of health‐promoting activities have been attributed to the colorless carotenoids. These include the quenching of hydroxyl radicals; activation of the antioxidant response element and thereby possible decreases in DNA damage in lymphocytes and reduction in inflammation. 55 Lumenato supplement has been shown to induce low levels of collagen‐3 that may contribute to skin protection by inhibiting free radicals secreted from neutrophils. 56 Phytoene and phytofluene absorb light maximally in the range of UV radiation (which explains why they appear colorless) protecting from both UVA‐ and UVB‐induced damage. An earlier study has demonstrated that UVB‐induced inflammation and immunosuppression activity is modulated by a tomato extract rich in multiple carotenoids including phytoene and phytofluene. 40 Products containing these actives have been deemed safe for human ingestion and topical use. 54
The study product is tomato oleoresin obtained from yellow dry tomato pulp in a high‐pressure process. While it does contain tomato components, it does not contain other known standard allergens. It is Kosher and Halal certified, non‐GMO, contains no animal products and is suitable for vegetarians. The product is provided at the dose of ~110 mg softgel. Analytic specifications indicate that the oleoresin contains 10%–12% of total carotenoids.
This study was designed to evaluate the perceived efficacy of an oral supplement (Lumenato™) containing a mix of carotenes in improving skin visual attributes after 12 weeks of use. Skin condition was assessed by validated tools (questionnaires). A total of 63 females completed the study. Participants were supplied with Lumenato supplement softgel every day for 12 weeks (84 days). The softgels were taken after a primary meal such as breakfast, lunch, or dinner.
The primary objective of this study was to assess the consumer‐perceived effectiveness of the Lumenato supplement through subjective skin health and appearance indicators as measured by the “FACE‐Q™ Satisfaction with Skin” scale at four time points (baseline, Week 4, Week 8, and Week 12) and then again two weeks after cessation of treatment.
3. MATERIALS
Lumenato supplement (Trade name: Lumenato); Oleoresin obtained from yellow tomato pulp. The supplement contains a mix of tomato carotenes predominantly phytoene and phytofluene, zeta‐carotene, and other naturally occurring tomato phytonutrients.
-
Part I: Plasma levels
The study was approved by the Helsinki Committee of the Soroka University Medical Center (approval No: 0012‐15‐SOR). The study was a multiple‐dose oral administration of the Lumenato capsules to healthy volunteers. Plasma phytoene, phytofluene, and zeta‐carotene levels were measured as a primary study outcome, and plasma lycopene levels (i.e., not contained in the Lumenato capsules) were used as an additional control.
Twenty‐four (24) healthy, nonsmoking volunteers, aged 24–36 years, were recruited to this study, out of which 5 were males and 18 females.
Subjects with liver or pancreatic disease, bowel disease, resection, abnormal fat metabolism, and those using medication suspected of interfering with fat‐soluble‐vitamin absorption, such as cholestyramine or aluminum hydroxide were excluded for the study. The subjects who consumed alcohol in excess of 40 g/d and those using multi‐vitamins or carotenoid supplements in the month preceding the study were also excluded.
The subjects came to the Endocrine Laboratory in a fasting state (overnight). The baseline blood sample was collected. Blood (11 ml) was collected using a butterfly needle and a vacuum tube (Vacutainer).
The subjects received individual packages of the Lumenato capsules for the 28‐day treatment period. 1, 2, 3, and 4 weeks later, the subjects came to the Endocrine Laboratory in a fasting state (overnight), the blood samples were collected, and subjects’ adherence to the experimental protocol was assessed by counting the unconsumed pills after the fourth week.
The collected blood samples were immediately placed in the dark and further sample handling was performed in subdued light. Plasma was separated and frozen at −70°C pending analysis of phytoene, phytofluene, lycopene, and zeta‐carotene. All plasma samples from an individual were analyzed together to minimize between‐analysis variability.
Plasma samples (0.5 ml) were mixed with an equal volume of ethanol and with astaxanthin as an internal standard (USP, standard lot# R006L0) and were extracted by hexane/dichloromethane (4/1), as described before. 57 The extract was collected, evaporated, and re‐dissolved in 0.2 ml 2‐propanol. The samples thus obtained were analyzed using a Dionex Ultimate 3000 UHPLC system including quaternary pump, degasser, column oven, autosampler, and PDA detector. Gradient elution was applied on a C18 reverse‐phase HPLC column in the multi‐wavelength mode. Mobile phase A: acetonitrile:methanol:ammonium acetate buffer = 25:25:50. Mobile phase B: acetonitrile:methanol:dichloromethane:hexane = 70:25:2.5:2.5. Gradient: 0 min—A 100%, 3.8 min—B 100%, 9.7 min—B 100%, 9.8 min—A 100%, and 14.0 min—A 100%. Lycopene, phytoene, phytofluene, and zeta‐carotene were in‐house standards. The reported lycopene concentrations were calculated from the sum of the lycopene isomers’ peak area.
Part II: Visual skin attributes
4. SUBJECTS
Healthy women between the ages of 35 and 55 interested in improving health and appearance of normal facial skin were recruited for the study. The subjects downloaded an app (ClaimIt, Obvio Health USA Inc.) which is an online and mobile app interface to execute clinical trials. This setup eliminates physical site visits and brings the trial directly to the mobile device of each subject.
The participants signed an electronic informed consent (eIC) before beginning the screening process after which they completed a study‐specific screening questionnaire with the inclusion/exclusion criteria, including self‐reported ethnicity and skin type. On qualification, they completed medical history and concomitant medication forms and enrolled in the study (Figure 1).
FIGURE 1.
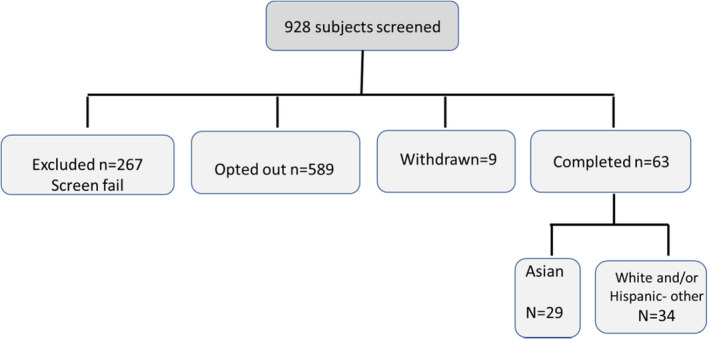
Subject recruitment chart
The qualified participants were shipped the study product. The participants took 1 softgel each day for 12 weeks around the time of a primary meal (breakfast, lunch, or dinner). They were required to complete questionnaires within the app as they are rolled out at predefined time points throughout the study.
The weekly e‐diary was used to measure compliance with consuming the study product as well as any reported changes in health or medication to determine adverse event if any. At the end of the study (12 weeks of use), the participants were instructed to safely discard any remaining softgels containing the study product and to complete a series of questionnaires relating to their experience with the study product.
Two weeks after completing the treatment period, the participants completed the FACE‐Q™ Satisfaction with Skin, End of Study Skin Questionnaire, and ClaimIt Experience Survey (Figure 2).
FIGURE 2.
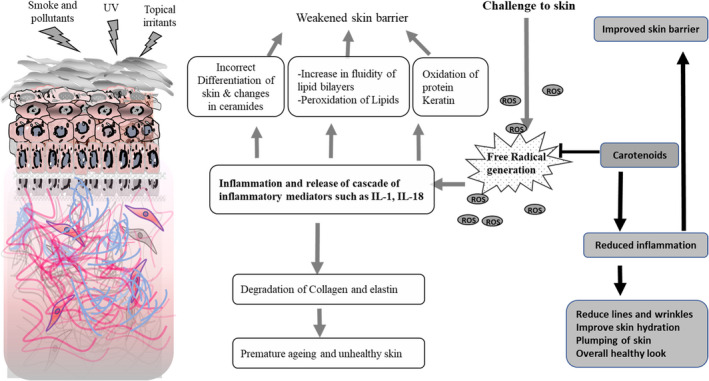
Skin is exposed to the environmental impact of challenges such as ultraviolet irradiation, smoke and pollutants as well as topical irritants. These challenges lead to generation of free radicals (ROS: reactive oxygen species) which can deplete the inherent reservoir of antioxidant system of skin thereby leading to release of a cascade of inflammatory mediators such as interleukins and arachidonic acid. Inflammatory response leads to various changes that disrupt the barrier including incorrect differentiation that changes ceramides of the stratum corneum, increase in fluidity of lipid bilayers as well as peroxidation of stratum corneum lipid and oxidation of stratum corneum proteins including keratin. The inflammatory response leads to degradation of collagen and elastin in the dermis which over time leads to unhealthy skin that looks prematurely aged. Treatment with carotenoids results in its accumulation in the skin, so it is available to quench the free radicals thereby preventing the inflammatory cascade from disrupting the barrier. Such protection leads to reduction in the aging process of skin thereby displaying a healthy look with reduced signs of age
5. STUDY ENDPOINTS PRIMARY ENDPOINT
Participant perception of the improvement in skin health and appearance from baseline to Week 12 using the questionnaire responses from FACE‐Q™ Satisfaction with Skin scale (baseline, Week 4, Week 8, and Week 12).
Adverse events (AEs) observed during the study, classified by the investigator as to severity, relationship to the study product/protocol, and seriousness.
Compliance to the daily use of the Lycored Lumenato supplement through monitoring within ClaimIt (weekly e‐diary).
Sustainability of effect after two weeks without supplementation as assessed by the Week 14 FACE‐Q™ Satisfaction with Skin scale and End of Study Skin Questionnaire.
5.1. Statistical considerations
A total of 72 female participants were recruited for the study out of which 63 completed the study. Nine subjects discontinued due to non‐product‐related reasons. Using Excel package statistical package, descriptive statistical analysis was performed on all parameters pertaining to the primary and secondary objectives.
Comparisons were made between baseline and 12‐week use. Skin update was administered at Weeks 4, 8, and 12 when the participants answered subjective skin health questionnaires using repeated measures ANOVA. Descriptive statistics (frequencies and percentages) summarized responses from the Study Product.
6. RESULTS
6.1. Part I
Plasma levels before and after multiple oral administration of Lumenato capsules (µM) are expressed in Figure 3. Baseline level of the carotenoids was consistent with published data. 46 Baseline level of phytoene was very low (0.07 µM); however, it increased significantly (p < 0.001), almost 4 times within the first week of treatment, and by 3–4 weeks, the plasma level of phytoene was 0.37–0.32 µM which is almost 5 times the baseline level. Similarly, the levels of phytofluene and zeta‐carotene also increased significantly after treatment with Lumeneto.
FIGURE 3.
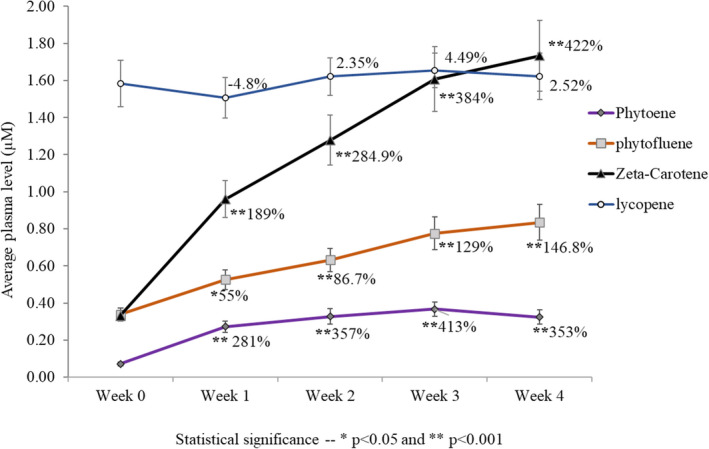
Plasma levels before and after multiple oral administrations of Lumeneto capsules (µM). Phytoene level increased significantly (p < 0.001); almost 4 times within the first week of treatment and by 3–4 weeks the plasma level of phytoene was 0.37–0.32 µM which is almost 5 times the baseline level. Phytofluene level in the plasma increased 55% (p < 0.05) within the first week of treatment and continued to increase significantly (p < 0.001) for the course of the study. After 4 weeks of use, there was about 2.5 times the baseline level. There was a marked increase in blood plasma level of zeta‐carotene after treatment; it reached over 5 times baseline after 4 weeks of treatment (p < 0.001). The baseline plasma level of lycopene was high (1.58 µM), and it did not change over the course of treatment
The baseline plasma level of lycopene was high (1.58 µM), and it did not change over the course of treatment since the treatment did not contain this active.
6.2. Part II: Visual skin attributes: Improvement scores
Figure 4 exhibits the average scores of different parameters before and after 12‐week use of the supplement. Figure 4A shows various measurements of skin tonality. As observed in the graph, although there was no improvement in pigmented spots, there was still a 21% improvement in evenness of skin tone (p < 0.001). The skin appeared 29.75% brighter (p < 0.001) and dark spots of the face reduced by 10.96% (p < 0.001). Periorbital dark circles also appeared to diminish by 17.9% (p < 0.001). Figure 4 exhibits the average scores of different parameters before and after 12‐week use of the supplement. As observed in Figure 4B, there was a highly significant (p < 0.001) improvement in skin elasticity (21.54%) and firmness (27.18%) after using the supplement for 12 weeks. Figure 4A shows various measurements of skin tonality. As observed in the graph, although there was no improvement in pigmented spots, there was still a 21% improvement in evenness of skin (p < 0.001). The skin appeared to be 29.75% brighter (p < 0.001) and dark spots of the face reduced by 10.96% (p < 0.001). Periorbital dark circles also appeared to diminish by 17.9% (p < 0.001).
FIGURE 4.
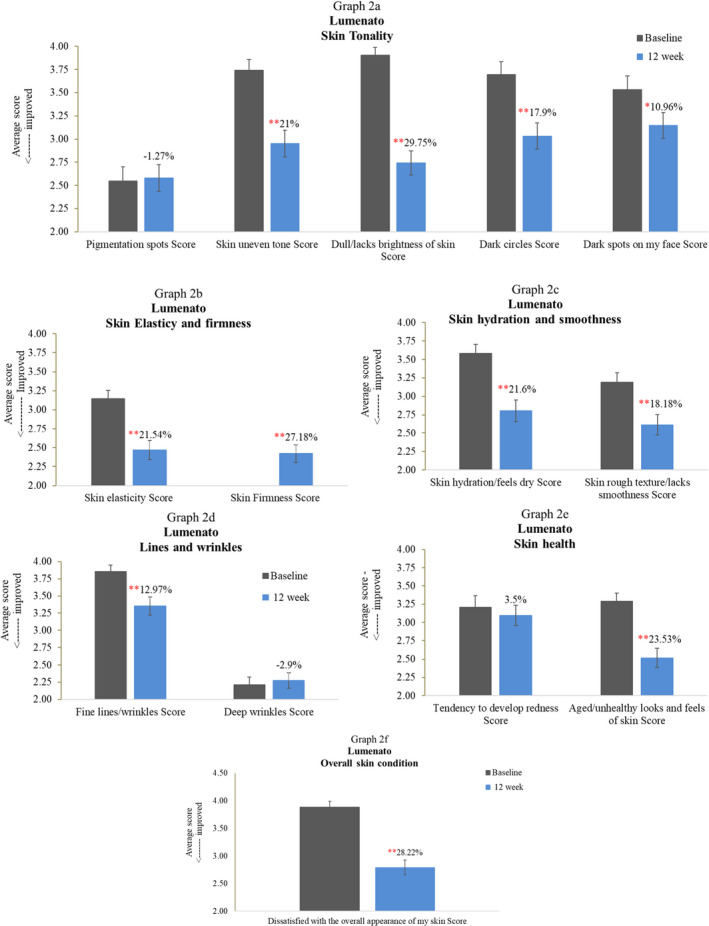
Exhibits the average scores of different parameters before and after 12‐week use of the supplement. As observed in (B) there was a highly significant (p < 0.001) improvement in skin elasticity (21.54%) and firmness (27.18%) after using the supplement for 12 weeks. (A) Shows various measurements of skin tonality. As observed in the graph, although there was no improvement in pigmented spots, there was still a 21% improvement in evenness of skin (p < 0.001) =. The skin appeared 29.75% brighter (p < 0.001), and dark spots of the face reduced by 10.96% (p < 0.001). Periorbital dark circles also appeared to diminish by 17.9% (p < 0.001). (C) Displays that there was 21.6% (p < 0.001) perceived improvement in skin hydration and 18.18% improvement in skin texture (p < 0.001) after 12 weeks of using the supplement. (D) Shows that although there was no improvement in deep wrinkles, there was a significant improvement of 12.97% (p < 0.001) in fine lines and wrinkles of the face after 12 weeks of supplement use. As observed in (E) there was a slight improvement in the tendency of the skin to develop redness. The general look of aged/unhealthy skin reduced significantly (p < 0.001) after using the supplement with a 23.53% reduction. (F) Shows the average score of overall skin condition before and after 12‐week treatment of the supplement. As observed in the graph, there was a highly significant (p < 0.001) improvement of 28.22% in the overall skin condition after using the supplement
Figure 4C displays that there was 21.6% (p < 0.001) perceived improvement in skin hydration and 18.18% improvement in skin texture (p < 0.001) after 12 weeks of using the supplement.
Figure 4D shows that although there was no improvement in deep wrinkles, there was a significant improvement of 12.97% (p < 0.001) in fine lines and wrinkles of the face after 12 weeks of supplement use. As observed in Figure 4E, there was a slight improvement in the tendency of the skin to develop redness. The general look of aged/unhealthy skin reduced significantly (p < 0.001) after using the supplement with a 23.53% reduction.
Figure 4F shows the average score of overall skin condition before and after 12 weeks of treatment of the supplement. As observed in the graph, there was a highly significant (p < 0.001) improvement of 28.22% in the overall skin condition after using the supplement.
Figure 5 exhibits results from FACE‐Q™ Satisfaction with Skin Scale before treatment and after 4, 8, and 12 weeks of treatment and again after treatment was stopped for 2 weeks. The data presented are from satisfaction score where score 1 was very dissatisfied, 2 was somewhat dissatisfied, 3 was somewhat satisfied, and 4 was very satisfied. The number of subjects reporting a score of 3 and 4 was counted and expressed in the graph as percent of population showing approval. Figure 5A shows “how does your facial skin look” first thing in the morning and at the end of the day. Initially, the score was at the edge of satisfied and dissatisfied; however, after treatment, there was a steady improvement up to eight weeks of 84%–75% (p < 0.001), after which there was a levelling off. The effect retained after the treatment was discontinued for two weeks. Figure 5B shows a similar trend for perception of healthy and attractive look of the face. For these both parameters, there was steady improvement over the course of 12‐week treatment at which point there was a statistically significant (p < 0.001) improvement of 74.6% and 65.08% (p < 0.001) for healthy and attractive skin, respectively. These perceptions continued to improve despite discontinued treatment for two weeks.
FIGURE 5.
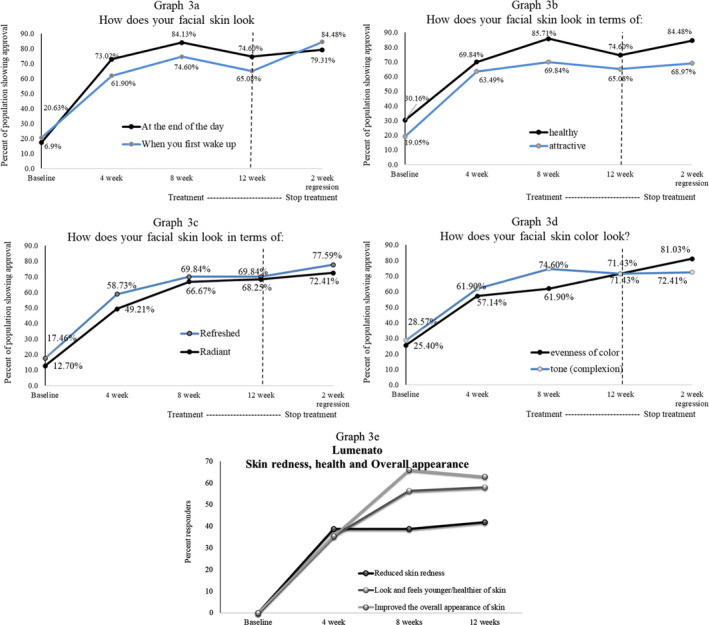
exhibits results from FACE‐QTM Satisfaction with Skin Scale before treatment and after 4, 8, and 12 weeks of treatment and again after treatment was stopped for 2 weeks. The data presented are from satisfaction score where score 1 was very dissatisfied, 2 was somewhat dissatisfied, 3 was somewhat satisfied, and 4 was very satisfied. The number of subjects reporting a score of 3 and 4 was counted and expressed in the graph as percent of population showing approval. (A) Shows “how does your facial skin look” first thing in the morning and at the end of the day. Initially, the score was at the edge of satisfied and dissatisfied; however, after treatment, there was a steady improvement up to eight weeks of 84%–75% (p < 0.001), after which there was a levelling off. The effect retained after the treatment was discontinued for two weeks. (B) Shows a similar trend for perception of healthy and attractive look of the face. For both these parameters, there was steady improvement over the course of 12‐week treatment at which point there was a statistically significant (p < 0.001) improvement of 74.6% and 65.08% (p < 0.001) for healthy and attractive skin, respectively. These perceptions continued to improve despite discontinued treatment for 2 weeks. (C) Perception of refreshed and radiant face. For these two parameters, there was steady improvement over the course of 12 weeks of treatment at which point there was a statistically significant (p < 0.001) change as compared to baseline. These perceptions continued to improve despite discontinued treatment for 2 weeks. (D) Facial skin tonality. After treatment there was a steady improvement up to eight weeks of 61.9%–74.6% (p < 0.001) in evenness of color and tonality. By the 12th week of treatment over 71% (p < 0.001) of the population professed that the treatment was effective for skin tonality and evenness of tone. After treatment was discontinued for two weeks, there was a further improvement of 81% and 72.41% in evenness of skin tone and tonality, respectively. (E) Shows that 38.71% of the population noticed a reduction in skin redness after using the supplement for 4 and 8 weeks, increasing to 41.94% after 12 weeks of supplement use. Improvement in the look and feel of healthy skin was observed by 35% of the population after 4 weeks of use increasing to 56.45% and 58.06% after 8‐ and 12‐week use, respectively. The overall appearance of skin improved in 35.48% of the subjects after 4 weeks of use increasing to 66.13% and 62.9% after 8 and 12 weeks, respectively
Figure 5C: Perception of refreshed and radiant face. For these two parameters, there was steady improvement over the course of 12 weeks of treatment at which point there was a statistically significant (p < 0.001) change as compared to baseline. These perceptions continued to improve despite discontinued treatment for two weeks.
Figure 5D: facial skin tonality. After treatment, there was a steady improvement up to eight weeks of 61.9%–74.6% (p < 0.001) in evenness of color and tonality. By the 12th week of treatment, over 71% (p < 0.001) of the population professed that the treatment was effective for skin tonality and evenness of tone. After treatment was discontinued for two weeks, there was a further improvement of 81% and 72.41% in evenness of skin tone and tonality, respectively.
Figure 5E shows that 38.71% of the population noticed a reduction in skin redness after using the supplement for 4 and 8 weeks, increasing to 41.94% after 12 weeks of supplement use. Improvement in the look and feel of healthy skin was observed by 35% of the population after 4 weeks of use increasing to 56.45% and 58.06% after 8 and 12‐week use, respectively.
The overall appearance of skin improved in 35.48% of the subjects after 4 weeks of use increasing to 66.13% and 62.9% after 8 and 12 weeks, respectively.
7. DISCUSSION
Skin is the main interface of an organism with its environment and skin structure, and function is affected by endogenous and environmental factors. Market is teeming with an array of topical products, and there is a growing awareness of the opportunity for using dietary supplements to optimize skin conditions. The skin requires an optimal supply of nutrition including antioxidant carotenoids, such as beta carotene, lycopene, as well as phytoene and phytofluene which have been reported to be useful nutritional supplements with photoprotective effects. 19 , 46 , 60 , 61 , 62 It has been suggested that under stress conditions, topical and/or systemic treatment with antioxidants contribute to skin moisturization, normal desquamation, overall balance of oxidative, and inflammatory status and thereby healthy, young‐looking skin. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 As observed in this study, the general look of aged/unhealthy skin reduced significantly (p < 0.001) after using the supplement for 12 weeks and was noticed by 54.84% of the population after 12 weeks of use. Antioxidants from diet or supplementation provide continuous nourishment for the skin, while with topical products, the maintenance of antioxidants in skin is local and transient. Lumenato has been shown to elevate collagen‐3 that may contribute to skin protection by inhibiting free radicals secreted from neutrophils. 56 Studies report that ingestion of carotenoids results in their increase in skin 48 , 49 ; thus, it can be inferred from this study that the Lumenato supplement accumulated in the skin and provided a prolonged benefit even after treatment was discontinued (as measured two weeks following the end of supplementation).
Carotenoids are dietary compounds of great interest in food science, nutrition, and health. Carotenoids like beta carotene are known to be converted into retinoids exhibiting vitamin‐A like anti‐aging activities in skin. As observed in this study, there was a consistent increase in plasma levels of carotenoids such as phytoene, phytofluene, and zeta‐carotene after ingesting Lumenato supplement. Carotenoids accumulate in skin where, besides promoting health by protecting from UV damage, they can provide cosmetic benefits by contributing to improved skin appearance and specific skin characteristics. 37 , 55 , 56
Phytoene has been reported to be a carotenoid with the highest bio‐accessibility followed by phytofluene. 58 The dietary source that provides highest amounts of potentially absorbable phytoene/phytofluene has been declared as tomato juice. 58 As observed in this study, the use of carotenoid supplement for 12 weeks significantly (p < 0.001) improved the appearance of skin elasticity, firmness, skin hydration, and texture as well as evenness of skin tone and facial brightness. These effects were noticed by close to 50% of the population. There was also a statistically significant reduction in facial dark spots of the face, and periorbital dark circles noticed by 32% and 38% of the population, respectively.
Facial lines and wrinkles are the most scrutinized manifestation of aging. It has been reported that the furrows and wrinkles are deeper and denser in the skin of individuals with a low antioxidant level. 48 In the current study, there was a significant reduction in the fine lines and wrinkles after 12 weeks of product use. Most subjects in this study exhibited a very low score for deep wrinkles; nevertheless, 61% of the subject population noticed an improvement in wrinkles after treatment with the supplement.
Daily ingestion of carotenoids for 12 weeks has shown to significantly increased serum level of lycopene and total skin carotenoids. Studies indicate that such increase is linked to improved protection from photodamage. 46 UV exposure increases skin inflammation; thus, it is possible that ingestion of the supplement reduced the noticeable skin inflammation due to the photoprotective effect of the carotenoids.
Studies have shown that administration of carotenoids elevates the levels of skin filaggrin protein which is instrumental in improving skin barrier. 59 A strengthened barrier further improves skin hydration, smoothness, and firmness elasticity as well as reduces fine lines and wrinkles. 10 , 23 Accumulation of carotenoids in the stratum corneum 35 possibly provides a prolonged benefit as a result of which that the subject self‐assessed improvement persisted after treatment was stopped.
AUTHOR CONTRIBUTIONS
K.H and E.T. involved in conceptualization, resources, review and editing, visualization, supervision, and funding acquisition. K.H., E.T., and Y.S involved in methodology. E.T. involved in investigation and project administration. N.M. involved in data analysis, writing, and artwork. All authors have read and agreed to the published version of the manuscript.
ETHICAL APPROVAL
This study was performed in accordance with the ethical principles based in the Declaration of Helsinki and its subsequent amendments, and in accordance with the International Council for Harmonization (ICH) Good Clinical Practice (GCP) guideline (ICH E6(R2), 2016), and applicable regulatory requirements. The study was approved by IntegReview IRB (IORG0000689), IntegReview Wednesday Board, Austin, Texas.
Tarshish E, Hermoni K, Sharoni Y, Muizzuddin N. Effect of Lumenato oral supplementation on plasma carotenoid levels and improvement of visual and experiential skin attributes. J Cosmet Dermatol. 2022;21:4042–4052. doi: 10.1111/jocd.14724
Funding information
This research received no external funding. The study was fully funded by the sponsor—Lycored
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Harman D. Free radical theory of aging. Mutat Res. 1992;275(3–6):257‐266. [DOI] [PubMed] [Google Scholar]
- 2. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47‐95. [DOI] [PubMed] [Google Scholar]
- 3. Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Aspects Med. 2003;24:345‐351. [DOI] [PubMed] [Google Scholar]
- 4. Zastrow L, Groth N, Klein F, et al. The missing link–light‐induced (280–1,600 nm) free radical formation in human skin. Skin Pharmacol Physiol. 2009;22:31‐44. [DOI] [PubMed] [Google Scholar]
- 5. Darvin ME, Haag SF, Lademann J, Zastrow L, Sterry W, Meinke MC. Formation of free radicals in human skin during irradiation with infrared light. J Invest Dermatol. 2010;130:629‐631. [DOI] [PubMed] [Google Scholar]
- 6. Taylor CR, Sober A. Sun exposure and skin disease. J. Annu Rev Med. 1996;47:181‐191. [DOI] [PubMed] [Google Scholar]
- 7. Dumay O, Karam A, Vian L, et al. Ultraviolet AI exposure of human skin results in Langerhans cell depletion and reduction of epidermal antigen‐presenting cell function: partial protection by a broad‐spectrum sunscreen. Br J Dermatol. 2001;144:1161‐1168. [DOI] [PubMed] [Google Scholar]
- 8. Cross CE, van der Vliet A, Louie S, Thiele JJ, Halliwell B. Oxidative stress and antioxidants at biosurfaces: plants, skin and respiratory tract surfaces. Environ Health Perspect. 1998;106(suppl 5):1241‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983;80:44‐49. [DOI] [PubMed] [Google Scholar]
- 10. Rawlings AV, Scott IR, Harding CR, Bowser PA. Stratum corneum moisturization at the molecular level. J Invest Dermatol. 1994;103:731‐740. [DOI] [PubMed] [Google Scholar]
- 11. Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220‐1224. [DOI] [PubMed] [Google Scholar]
- 12. Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313‐20316. [DOI] [PubMed] [Google Scholar]
- 13. Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age‐related changes in oxidized proteins. J Biol Chem. 1987;262:5488‐5491. [PubMed] [Google Scholar]
- 14. Broekaert D, Cooreman K, Coucke P, et al. A quantitative histochemical study of sulphydryl and disulphide content during normal epidermal keratinization. Histochem J. 1982;14:573‐584. [DOI] [PubMed] [Google Scholar]
- 15. Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1‐11. [DOI] [PubMed] [Google Scholar]
- 16. Melov S. Mitochondrial oxidative stress. Physiologic consequences and potential for a role in aging. Ann N Y Acad Sci. 2000;908:219‐225. [DOI] [PubMed] [Google Scholar]
- 17. Viña J, Borras C, Abdelaziz KM, Garcia‐Valles R, Gomez‐Cabrera MC. The free radical theory of aging revisited: the cell signaling disruption theory of aging. Antioxid Redox Signal. 2013;19(8):779‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sies H, Stahl W. Carotenoids and UV protection. Photochem Photobiol Sci. 2004;3:749‐752. [DOI] [PubMed] [Google Scholar]
- 19. Krutmann J. Inhibitory effects of sunscreens on the development of skin cancer. Hautarzt. 2001;52:62‐63. [DOI] [PubMed] [Google Scholar]
- 20. Moon JS, Oh CH. Solar damage in skin tumors: quantification of elastotic material. Dermatology. 2001;202:289‐292. [DOI] [PubMed] [Google Scholar]
- 21. Fuchs J. Potentials and limitations of the natural antioxidants RRR‐alpha‐tocopherol, L‐ascorbic acid and beta‐carotene in cutaneous photoprotection. Free Radic Biol Med. 1998;25:848‐873. [DOI] [PubMed] [Google Scholar]
- 22. Sander CS, Chang H, Hamm F, Elsner P, Thiele JJ. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int J Dermatol. 2004;43:326‐335. [DOI] [PubMed] [Google Scholar]
- 23. Thiele J, Schroeter C, Hsieh SN, Podda M, Packer L. The antioxidant network of the stratum corneum. Curr Probl Dermatol. 2001;29:26‐42. [DOI] [PubMed] [Google Scholar]
- 24. Berson DS. Natural antioxidants. J Drugs Dermatol. 2008;7:7‐12. [PubMed] [Google Scholar]
- 25. Dal Belo SE, Gaspar LR, Maia Campos PM, Marty JP. Skin penetration of epigallocatechin‐3‐gallate and quercetin from green tea and Ginkgo biloba extracts vehiculated in cosmetic formulations. Skin Pharmacol Physiol. 2009;22:299‐304. [DOI] [PubMed] [Google Scholar]
- 26. Verstraeten SV, Hammerstone JF, Keen CL, Fraga CG, Oteiza PI. Antioxidant and membrane effects of procyanidin dimers and trimers isolated from peanut and cocoa. J Agric Food Chem. 2005;53:5041‐5048. [DOI] [PubMed] [Google Scholar]
- 27. Kenny TP, Keen CL, Jones P, Kung HJ, Schmitz HH, Gershwin ME. Cocoa procyanidins inhibit proliferation and angiogenic signals in human dermal microvascular endothelial cells following stimulation by low‐level H2O2. Exp Biol Med (Maywood). 2004;229:765‐771. [DOI] [PubMed] [Google Scholar]
- 28. Mulero M, Rodriguez‐Yanes E, Nogues MR, et al. Polypodium leucotomos extract inhibits glutathione oxidation and prevents Langerhans cell depletion induced by UVB/UVA radiation in a hairless rat model. Exp Dermatol. 2008;17:653‐658. [DOI] [PubMed] [Google Scholar]
- 29. Thiele JJ, Dreher F, Packer L. Antioxidant defense systems in skin. In: Elsner P, Maibach H, eds. Drugs vs Cosmetics: Cosmeceuticals?. Dekker; 2000:145‐188. [Google Scholar]
- 30. Shindo Y, Witt E, Han D, Epstein W, Packer L. Enzymic and non‐enzymic antioxidants in epidermis and dermis of human skin. J Invest Dermatol. 1994;102:122‐124. [DOI] [PubMed] [Google Scholar]
- 31. Thiele JJ, Traber MG, Packer L. Depletion of human stratum corneum vitamin E: an early and sensitive in vivo marker of UV‐induced photooxidation. J Invest Dermatol. 1998;110:756‐761. [DOI] [PubMed] [Google Scholar]
- 32. Weber SU, Thiele JJ, Packer L, Cross CE. Vitamin C, uric acid and glutathione gradients in murine stratum corneum and their susceptibility to ozone exposure. J Invest Dermatol. 1999;113:1128‐1132. [DOI] [PubMed] [Google Scholar]
- 33. Bommannan D, Potts RO, Guy RH. Examination of stratum corneum barrier function in vivo by infrared spectroscopy. J Invest Dermatol. 1990;95:403‐408. [DOI] [PubMed] [Google Scholar]
- 34. Pelle E, Muizzuddin N, Mammone T, Marenus K, Maes D. Protection against endogenous and UVB‐induced oxidative damage in stratum corneum lipids by an antioxidant‐containing cosmetic formulation. Photoderm Photoimmunol Photomed. 1999;15:115‐119. [DOI] [PubMed] [Google Scholar]
- 35. Black HS, Mathews‐Roth MM. Protective role of butylated hydroxytoluene and certain carotenoids in photocarcinogenesis. Photochem Photobiol. 1991;53:707‐716. [DOI] [PubMed] [Google Scholar]
- 36. Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532‐538. [DOI] [PubMed] [Google Scholar]
- 37. Tarshish E, Hermoni K, Schwartz SR. Effect of oral supplement “lycopene” on reducing the signs of skin ageing. Clin Pharmacol Biopharm. 2020;9(2):195. [Google Scholar]
- 38. Ribaya‐Mercado JD, Garmyn M, Gilchrest BA, Russel RM. Skin lycopene is destroyed preferentially over beta‐carotene during ultraviolet irradiation in humans. J Nutr. 1995;125:1854‐1859. [DOI] [PubMed] [Google Scholar]
- 39. Biesalski HK, Hemmes C, Hopfenmuller W, Schmid C, Gollnick JP. Effects of controlled exposure of sunlight on plasma and skin levels of beta‐carotene. Free Radic Res. 1996;24:215‐224. [DOI] [PubMed] [Google Scholar]
- 40. Groten K, Marini A, Grether‐Beck S, et al. Tomato phytonutrients balance UV response: results from a double‐blind, randomized, placebo‐controlled study. Skin Pharmacol Physiol. 2019;32:101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Canene‐Adams K, Campbell JK, Zaripheh S, Jeffery EH, Erdman JW Jr. The tomato as a functional food. J Nutr. 2005;135:1226‐1230. [DOI] [PubMed] [Google Scholar]
- 42. Giovannucci E. Tomato products, lycopene, and prostate cancer: a review of the epidemiological literature. J Nutr. 2005;135:S2030‐S2031. [DOI] [PubMed] [Google Scholar]
- 43. Etminan M, Takkouche B, Caamano‐Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta‐anal ysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2004;13:340‐345. [PubMed] [Google Scholar]
- 44. Borel P, Moussa M, Reboul E, et al. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J Nutr. 2007;137:2653‐2659. [DOI] [PubMed] [Google Scholar]
- 45. Yeum KJ, Booth SL, Sadowski JA, et al. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1996;64(4):594‐602. [DOI] [PubMed] [Google Scholar]
- 46. Aust O, Stahl W, Sies H, Tronnier H, Heinrich U. Supplementation with tomato‐based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV‐light‐induced erythema. Int J Vitam Nutr Res. 2005;75:54‐60. [DOI] [PubMed] [Google Scholar]
- 47. Parker RS. Carotenoids in human blood and tissues. J Nutr. 1989;119(1):101‐104. [DOI] [PubMed] [Google Scholar]
- 48. Lademann J, Meinke MC, Sterry W, Darvin ME. Carotenoids in human skin. Exp Dermatol. 2011;20(5):377‐382. [DOI] [PubMed] [Google Scholar]
- 49. Cutler RG. Antioxidants and aging. Am J Clin Nutr. 1991;53(1 Suppl):373S‐379S. [DOI] [PubMed] [Google Scholar]
- 50. Walfisch Y, Walfisch S, Agbaria R, Levy J, Sharoni Y. Lycopene in serum, skin and adipose tissues after tomato oleoresin supplementation in patients undergoing haemorrhoidectomy or fistulotomy. Br J Nutr. 2003;90:759‐766. [DOI] [PubMed] [Google Scholar]
- 51. Salter D. Non‐invasive cosmetic efficacy testing in human volunteers: some general principles. Skin Res and Technol. 1996;2(2):59‐63. [DOI] [PubMed] [Google Scholar]
- 52. Nobile V. Guidelines on cosmetic efficacy testing on humans. Ethical, technical, and regulatory requirements in the main cosmetics markets. J Cosmo Trichol. 2016;2:1‐10. [Google Scholar]
- 53. Meléndez‐Martínez AJ, Mapelli‐Brahm P, Benítez‐González A, Stinco CM. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Arch Biochem Biophys. 2015;572:188‐200. [DOI] [PubMed] [Google Scholar]
- 54. Havas F, Krispin S, Meléndez‐Martínez AJ, von Oppen‐Bezalel L. Preliminary data on the safety of phytoene‐ and phytofluene‐rich products for human use including topical application. J Toxicol. 2018;2018:5475784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meléndez‐Martínez AJ, Mapelli‐Brahm P, Stinco CM. The colourless carotenoids phytoene and phytofluene: from dietary sources to their usefulness for the functional foods and nutricosmetics industries. J Food Composit Anal. 2018;67:91‐103. [Google Scholar]
- 56. Solomonov Y, Hadad N, Pikovsky O, Levy R. Lumenato protects normal human dermal fibroblasts from neutrophil‐induced collagen‐3 damage in co‐cultures. PLoS One. 2021;16(3):e0248183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stahl W, Sundquist AR, Hanusch M, Schwarz W, Sies H. Separation of beta‐carotene and lycopene geometrical isomers in biological samples. Clin Chem. 1993;39:810‐814. [PubMed] [Google Scholar]
- 58. Roberts WE. Skin type classification systems old and new. Dermatol Clin. 2009;27(4):529‐533. [DOI] [PubMed] [Google Scholar]
- 59. Mapelli‐Brahm P, Corte‐Real J, Meléndez‐Martínez AJ, Bohn T. Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chem. 2017;229:304‐311. [DOI] [PubMed] [Google Scholar]
- 60. Kake T, Imai M, Takahashi N. Effects of β‐carotene on oxazolone‐induced atopic dermatitis in hairless mice. Exp Dermatol. 2019;28(9):1044‐1050. [DOI] [PubMed] [Google Scholar]
- 61. Heinrich U, Gärtner C, Wiebusch M, et al. Supplementation with beta‐carotene or a similar amount of mixed carotenoids protect humans from UVinduced erythema. J Nutr. 2003;133:98‐101. [DOI] [PubMed] [Google Scholar]
- 62. Stahl W, Heinrich U, Jungmann H, Sies H, Tronnier H. Carotenoids and carotenoids plus vitamin E protect against UV‐light induced erythema in humans. Am J Clin Nutr. 2000;71:795‐798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


