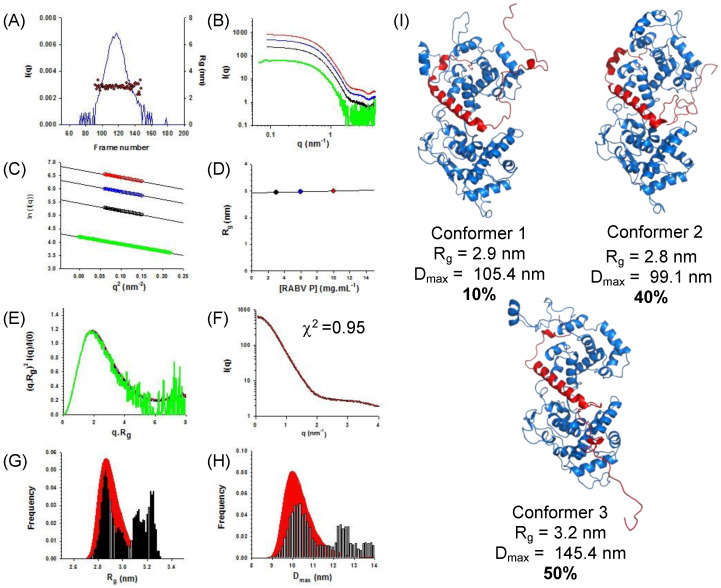
Figure 3.
SAXS and SEC-SAXS of RABV N∆230−P68 core complex. (A) SEC-SAXS elution profile and Rg across the elution peak; 50 μL of the N∆230−P68 sample were injected onto a Superdex 200 column and monitored on-line by SAXS. The black line shows the intensity at zero angle (I0), which is proportional to both the MM and concentration. The red dots indicate the values of the radius of gyration calculated from the Guinier approximation at the different time intervals. (B) SAXS profiles at different protein concentrations. SAXS profiles were recorded in batch mode at 3 mg.mL−1 (in black), 6 mg.mL−1 (in blue), and 11 mg.mL−1 (in red). The curve in green was obtained by averaging the individual profiles recorded throughout the SEC elution peak shown in Panel (A). (C) Guinier plots at different protein concentrations. Same color scheme as in panel (B). (D) Rg at different protein concentrations. The Rg value were calculated from the Guinier plot (panel (C)) for the profiles recorded in batch mode. (E) Dimensionless Kratky plots at different protein concentrations. Same color scheme as in panel (B). (F) Merged curve and conformational ensemble modeling by the ensemble optimization method (EOM). The black line shows the scattering profile obtained by merging segments of the profiles obtained at different protein concentrations (panel (B)). The red line shows a back-calculated scattering curve for a selected ensemble of three conformers measured in different proportions and shown in panel (I) (χ2 = 0.95). (G) Rg distribution. The red area shows the Rg distribution calculated for the initial ensemble of conformers, whereas the black bars show the Rg distribution of the selected ensemble that fit the experimental SAXS data (panel (F)). (H) Dmax distribution. The red area shows the Dmax distribution calculated for the initial ensemble of conformers, whereas the black bars show the Dmax distribution of the selected ensemble that fit the experimental SAXS data. (I) Representative ensemble of conformers. N is shown in blue and P68 including the eight C-terminal residues of the linker and His-tag are shown in red (cartoon representation).