Summary
The periodontal complex involves the hard and soft tissues which support dentition, comprised of cementum, bone, and the periodontal ligament (PDL). Periodontitis, a prevalent infectious disease of the periodontium, threatens the integrity of these tissues and causes irreversible damage. Periodontal therapy aims to repair and ultimately regenerate these tissues toward preserving native dentition and improving the physiologic integration of dental implants. The PDL contains multipotent stem cells, which have a robust capacity to differentiate into various types of cells to form the PDL, cementum, and alveolar bone. Selection of appropriate growth factors and biomaterial matrices to facilitate periodontal regeneration are critical to recapitulate the physiologic organization and function of the periodontal complex. Herein, we discuss the current state of clinical periodontal regeneration including a review of FDA‐approved growth factors. We will highlight advances in preclinical research toward identifying additional growth factors capable of robust repair and biomaterial matrices to augment regeneration similarly and synergistically, ultimately improving periodontal regeneration's predictability and long‐term efficacy. This review should improve the readers' understanding of the molecular and cellular processes involving periodontal regeneration essential for designing comprehensive therapeutic approaches.
Keywords: neural crest, periodontology, teeth, tissue, tissue engineering
1. INTRODUCTION TO REGENERATIVE MEDICINE IN PERIODONTOLOGY
The periodontal complex involves the hard and soft tissues which support dentition. Given its significant roles in force transduction, providing sensory information about tooth position, facilitating jaw reflexes during tooth movement, and resistance to mechanical forces, and the prevalence of periodontal disease, regeneration of the periodontium is a significant and complex clinical challenge (Zhao, Volponi, Caetano, & Sharpe, 2020). The goal of tissue engineering and regenerative medicine is to repair and replace damaged tissues in the course of the disease, to restore physiologic function (Hoffman, Khademhosseini, & Langer, 2019; Langer & Vacanti, 1993, 2016). In the case of periodontal tissues, the field of periodontology has made significant progress in translating periodontal regeneration techniques to the patient care (Galli, Yao, Giannobile, & Wang, 2021; Giannobile & McClain, 2015; Menicanin, Hynes, Han, Gronthos, & Bartold, 2015). While considerable progress has been made in areas of alveolar bone and gingival soft tissue regeneration, these treatments have had limited success in recapitulating a regenerated periodontal ligament's (PDL) structural and functional aspects. This review does not cover perspectives on the potential regeneration of teeth, but we encourage interested readers to reference El Gezawi, Wölfle, Haridy, Fliefel, and Kaisarly (2019) and W. Zhang and Yelick, (2021). This review aims to highlight advances in periodontal regeneration, emphasizing the need for future development to increase the predictability and outcomes of PDL regeneration informed by recent advances in understanding its developmental origins.
Tissue engineering aims to recapitulate three‐dimensional (3D) tissue structure by bringing together cells capable of regeneration with an environment adequate for tissue neogenesis, including both physical and chemical inductive signals (Chan & Leong, 2008). These crucial components are referred to as the tissue engineering triad and are essential in regenerative strategies' design and clinical implementation (Figure 1).
Biomaterials Scaffold: Biomaterial scaffolds provide a 3D environment that organizes cells in three dimensions (Swanson & Ma, 2020); recent advances in biomaterials engineering and mechanobiology point to the role of biomaterials' physical morphology and mechanical properties as crucial design motifs in determining the trajectory of cells in engineered microenvironments (Gupte et al., 2018; Naqvi & McNamara, 2020; Swanson et al., 2021; Y. Zhang et al., 2017). Biomaterials must be biocompatible to prevent host rejection and biodegradable at a rate that facilitates replacement of the degrading scaffold with new tissue without loss of engineered tissue integrity (Langer & Vacanti, 1993, 2016). Biomaterial scaffolds may be combined with drug delivery systems for sustained and controlled delivery of inductive signals to the tissue defect compartment.
Regenerative cell population: Cells capable of regeneration may be endogenous cells that migrate to a defect site or may be transplanted (Langer & Vacanti, 1993). Several kinds of stem cells have been explored as candidates for their usefulness in regenerating dental tissue, including dental pulp stem cells (Rubins, Tolmie, Corsig, Kerr, & Kim, 2014), stem cells from the apical papilla (Kang, Fan, Deng, He, & Huang, 2019), dental follicle precursor cells (Guo et al., 2012), PDL stem cells (Queiroz et al., 2021), alveolar bone stem cells (Tan et al., 2009), and stem cells from human exfoliated deciduous teeth (SHED) (Gao et al., 2018).
Inductive signals: Inductive signals increase the predictability and specificity of regenerative trajectories (Ren, Zhao, Lash, Martino, & Julier, 2020). Most often, inductive signals are growth factors and morphogens but may also be small molecule and peptide therapeutics or genes and other bioactive molecules. Similarly, inductive signals may come from cells surrounding a defect or secreted by transplanted or newly migrated cells (Kim, Hong, & Son, 2016). Biomaterial matrices may provide tissue inductive signals by promoting specific differentiation fates of cells through engineered physical, chemical, and mechanical stimuli (Rambhia & Ma, 2015).
FIGURE 1.
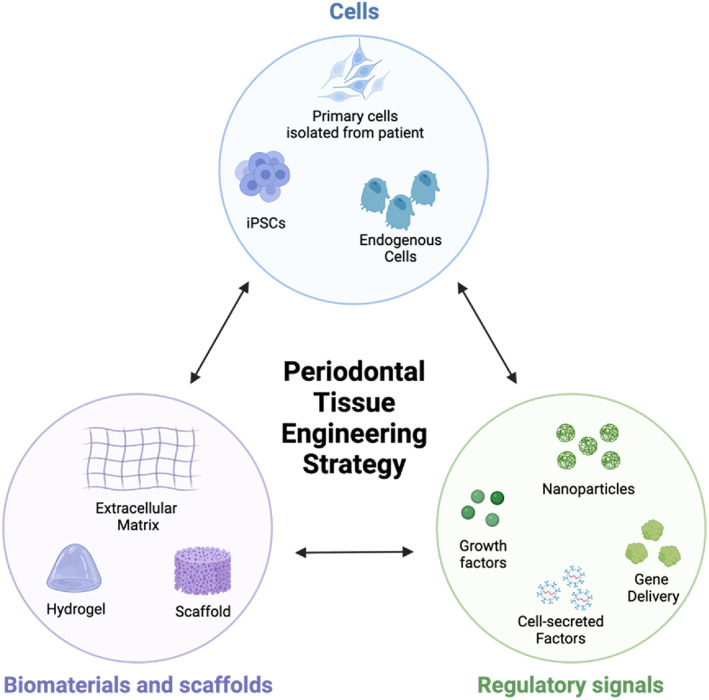
The tissue engineering triad illustrates the three critical design criteria for tissue engineering strategies. Figure prepared with Biorender
This highly interdisciplinary approach to treating disease involves recapitulating tissue and organ formation and relies significantly on recent findings from developmental biology, stem cell biology, advanced tissue culture techniques, and biomaterials engineering.
2. CLINICAL SIGNIFICANCE OF THE PDL IN HEALTH AND DISEASE
The PDL is a dynamic and biomechanically active craniofacial fibrous joint that provides a direct interface between teeth and the supporting alveolar bone (Figure 2a) (Chiego, 2018; Ten Cate, 1998). The PDL serves to attach teeth to the alveolar bone. In doing so, it facilitates tooth displacement within the bony socket where teeth reside and acts to dampen and distribute cyclic masticatory forces. It sustains tissue remodeling in response to mechanical forces in the cementum and bone (McCulloch, Lekic, & McKee, 2000).
FIGURE 2.
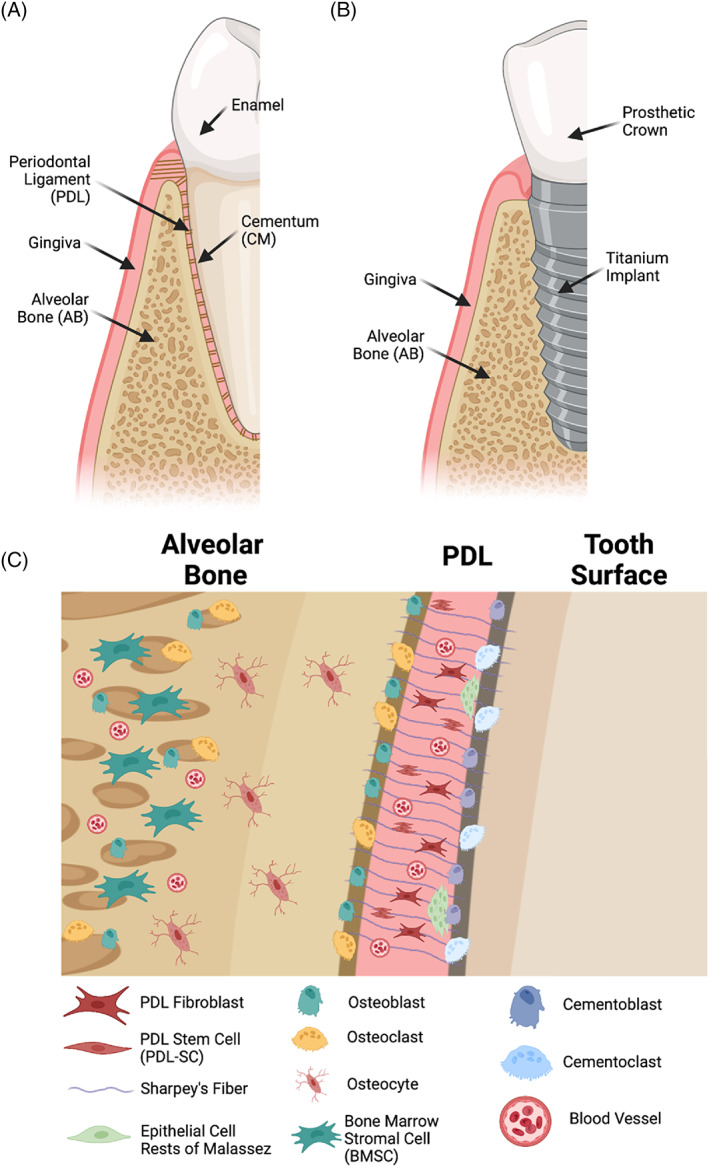
Schematic overview of periodontal tissues in the case of native dentition (a) and dental implants (b). The physiologic periodontium involves a complex milieu of cell types between alveolar bone, the periodontal ligament, and cementum (c). Figure prepared with Biorender
The PDL comprises bundles of parallel collagen fibers and elastin, which are highly ordered to withstand, respond to, and transmit masticatory loads (S. H. Liu, Yang, Al‐Shaikh, & Lane, 1995). The PDL comprises collagen Type I but also contains Types V and VI, chondroitin sulfate, proteoglycans, fibronectin, tenascin, and undulin. These fibers insert into the alveolar bone and cementum as Sharpey's fibers, which serve as anchoring points. Interestingly, McKee, Zalzal, and Nanci (1996) demonstrated that Sharpey's fibers accumulate high levels of osteopontin at their insertion site in alveolar bone, but not in cementum, among other non‐collagenous proteins with distinct spatial organization within the PDL. The PDL is richly vascularized with blood vessels that communicate with those from the gingiva, alveolar bone, and apical region of teeth, with a standard pattern of occlusal‐apical orientation (Masset, Staszyk, & Gasse, 2006). The PDL also contains dense innervations by myelinated nerve fibers near collagen fibers and the alveolar bone (Y. Huang, Corpas, Martens, Jacobs, & Lambrichts, 2011).
Based on the observation that the PDL is maintained throughout the highly variable applied loads experienced in normal masticatory function, the PDL requires a highly effective system for sensing, maintaining, and adapting its role. Periodontal disease involves the loss of tooth‐supporting structures; its hallmark feature is the loss of alveolar bone (Tonetti, Greenwell, & Kornman, 2018). Along with alveolar bone loss is the loss of the bone‐integrated support structure—the PDL. Periodontitis is the 11th most common disease worldwide and is identified by the US Surgeon General's Report on Oral Health as a critical challenge to human health and the disease (Kassebaum et al., 2017). Therefore, periodontal disease represents a significant healthcare burden (Botelho et al., 2022), and developing regenerative treatments is a meaningful and vital opportunity. Loss of the PDL may also result in a manifestation of systemic disease, as reviewed by Albandar, Susin, and Hughes (2018). These systemic diseases largely influence periodontal inflammation, notably obesity, osteoporosis, diabetes mellitus, and HIV infection. Independent of periodontitis, odontogenic tumors and neoplasms of periodontal tissue, scleroderma, Langerhans cell histiocytosis, and giant cell granulomas can result in loss of periodontal tissue.
Dental implants replace dentition lost to trauma or disease as an implantable prosthetic. In contrast to native dentition, titanium dental implants directly integrate with the alveolar bone (Figure 2b) (Buser, Sennerby, & De Bruyn, 2000). Osseointegration has been demonstrated to be critical for implant stability and long‐term clinical success (Rutkowski, 2018). Collagen fibers in the surrounding alveolar bone are oriented parallel to the implant surface. They do not attach to the implant, unlike PDL fibers oriented perpendicular to Sharpey's fibers attachments into the cementum and bone (Tete, Mastrangelo, Bianchi, Zizzari, & Scarano, 2009; Traini, Degidi, Strocchi, Caputi, & Piattelli, 2005). We believe this represents an exciting opportunity for innovation, which will be discussed in further sections.
The PDL contains and interfaces with several distinct cell populations (Figure 2c). The predominant cell type is the fibroblasts (McCulloch & Bordin, 1991). Fibroblasts are principally responsible for collagen turnover and maintenance within the PDL (Everts, van der Zee, Creemers, & Beertsen, 1996). PDL fibroblasts activated by mechanical forces secrete plasminogen activator and matrix metalloproteases, as well as their inhibitors, cytokines (i.e., PGE‐2), and interleukin‐6 (Lekic, Rajshankar, Chen, Tenenbaum, & McCulloch, 2001). There is spatial, developmental, and functional heterogeneity among PDL fibroblasts (Freeman & ten Cate, 1971; Groeneveld, Everts, & Beertsen, 1993; McCulloch & Bordin, 1991).
Alveolar bone and cementum interface with the PDL. Osteoblasts and osteoclasts on the surface of alveolar bone have anabolic and resorptive functions, respectively, are responsible for bone turnover and adaptation, and are also involved in orthodontic tooth movement (N. Jiang et al., 2016). Similarly, cementoblasts and cementoblasts in the cementum are responsible for anabolic and resorptive functions, respectively. Epithelial cell rests of Malassez are also found in the PDL and are quiescent epithelial remnants from Hertwig's epithelial root sheath, involved in the formation of tooth roots (Shinmura, Tsuchiya, Hata, & Honda, 2008). They play a role in the cementum and enamel repair (Tsunematsu et al., 2016). Endothelial cells form the lining of blood vessels.
3. PERSPECTIVE ON PDL REGENERATION
Following insult by trauma or disease, the ideal clinical outcome would be the regeneration of the periodontal support apparatus, including alveolar bone, cementum, and PDL. New attachment, therefore, involves the regeneration of the principle fibers which compose the PDL and their reattachment into newly formed cementum on the root surface. This process, performed partly by PDL cells, competes with apical migration of the pocket‐lining junctional epithelium (Wikesjo, Sigurdsson, Lee, Tatakis, & Selvig, 1995). Nyman et al. were among the first to demonstrate that a new PDL can be established on a previously diseased root surface (Nyman, Lindhe, Karring, & Rylander, 1982). Their seminal study in a human patient showed that cells originating from the PDL might migrate coronally to a root surface previously exposed to a periodontal pocket to deposit new cementum and attachment fibers when adequately isolated from the sulcular epithelium with a cellulose acetate membrane. Furthermore, they demonstrated that the formation of new connective tissue attachment is not necessarily accompanied by coronal regeneration of alveolar bone.
The PDL contains a self‐renewing progenitor cell population, identified as the PDL stem cell (PDL‐SC), found in perivascular spaces of the periodontium (McCulloch, Barghava, & Melcher, 1989; McCulloch & Melcher, 1983; Seo et al., 2004). This stem cell population is critical to the PDL's ability to adapt and self‐renew throughout life. PDL stem cells obtained from mature PDL of erupted teeth possess similar stem cell properties to mesenchymal stem cells (MSCs) (Kaku et al., 2012). Their molecular characteristics are summarized by Zhu and Liang (2015). These cells are characterized by a rapid turnover rate and distinct localization near blood vessels in the PDL and are capable of osteogenic, cementogenic, and fibroblastic differentiation fates (Palmer & Lumsden, 1987). Compared to bone marrow mesenchymal stem cells (BMSCs) often used in preclinical models of craniofacial regeneration, both BMSC and PDL‐SCs are capable of robust bone regeneration, but only PDL‐SCs show a favorable effect on PDL formation (Yan, Yang, Jansen, de Vries, & van den Beucken, 2015). Their study is significantly important to PDL regeneration and is an area of active investigation.
PDL‐SCs are a key cell source to harness in PDL regeneration. Importantly, they are present in human adults throughout life. However, their number decreases with patient age (Zheng et al., 2009) and can be expanded ex vivo (Seo et al., 2004) as well as recovered from cryopreservation (Seo et al., 2005). In the context of autologous use, PDL‐SCs from adults older than 41 years demonstrated decreased regenerative capacity. Growth factor treatment, optimized growth factor administration, or biomaterials capable of stem cell expansion are exciting. Allogenic use, rather than autologous, may also be possible. MSCs from the gingiva may be more easily accessible for cell isolation when tooth extraction is not indicated. They have been demonstrated to regenerate cementum, alveolar bone, and PDL in a canine model (Seo et al., 2005) and may be more resistant to the effects of inflammation than PDL‐SCs (Yang et al., 2013). In vitro models of both PDL‐SCs and GMSCs will allow for a high throughput screening platform of nanoparticle, small molecule, and protein therapeutics and provide important insight into their molecular behavior.
Yan, Yang, et al. (2015) summarized the state of periodontal tissue regeneration in preclinical animal models in a 2015 meta‐analysis, which provided significant insight into regenerative outcomes and cell sources. They included 39 studies fitting their meta‐analysis criteria. PDL‐derived cells and BMSCs are equally efficacious in inducing new bone formation; differences in cementum formation capacity were not statistically significant. Interestingly, only PDL‐SCs show a favorable effect on PDL formation. Like BMSCs, PDL‐SCs have been demonstrated to possess unique immunomodulatory properties, which may be advantageous for cell transplantation therapies (Wada, Menicanin, Shi, Bartold, & Gronthos, 2009), summarized by Wada et al.
Feng et al. (2010) provided an early report in which periodontal defects were treated with autologous PDL progenitor population. PDL‐SCs were isolated from extracted third molars; the PDL tissue was separated from the tooth, and cells were cultured on a calcite surface resulting in a cell sheet. The calcite/PDL‐SC sheet was inserted into a mucoperiosteal flap following debridement. While limited by patient number (n = 3), the authors described significant decreases in probing depth and mobility and increased clinical attachment and radiographic bone fill. F.‐M. Chen et al. (2016) report an early small‐scale randomized clinical trial demonstrating the utility of autologous PDL‐SCs delivered in combination with a bone xenograft material (Bio‐oss) to regenerate alveolar bone height and clinical attachment with no adverse events. These trials serve as preliminary evidence for the potential of PDL‐SCs as valuable agents of PDL regeneration in the broader context of periodontal regeneration. We will summarize the current state of the art in periodontal regeneration and revisit this evidence to date, considering its future development.
Significant excitement in periodontal regeneration has been generated in both preclinical and clinical literature. Multiple stem cell technologies have been successfully validated in human clinical trials to regenerate dental/oral tissues. Furthermore, in various small sample studies, clinicians' attitudes (Aiyegbusi et al., 2020; y Baena, Casasco, & Monti, 2022) and medical and dental students (Burdick, Mauck, Gorman, & Gorman, 2013) are positive toward cell‐based regenerative technologies. These surveys also demonstrate poor knowledge of the use of stem cells and a need for significant clinician education to enable their efficient clinical adoption (y Baena et al., 2022). Interestingly, the opinions of dental hygienists and assistants, among other clinical auxiliary staff, have not been well reported. These individuals are key players in the patient care team and will likely play a significant role in the clinical workflow related to stem cell‐based technology. Furthermore, patient education and public perception will require appropriate education on various aspects of the new medical technology (Aiyegbusi et al., 2020). Biomaterials and growth factors that take advantage of endogenous cell sources, rather than cell transplantation, may represent a more near‐term technology achievement in the periodontal regeneration (Burdick et al., 2013); mechanical debridement procedures highlight the endogenous regenerative capacities of the periodontium, which exogenous factors can further augment.
4. CURRENT PROGRESS IN PERIODONTAL REGENERATION
Scaling and root planing (SRP) remains a gold standard non‐surgical treatment for periodontal defects by the mechanical removal of disease‐causing agents (Herrera, 2016). The primary goal in removing subgingival calculus, biofilm deposits, and diseased cementum is to create a root surface capable of reattachment with periodontal tissues. SRP, along with oral hygiene instruction for home care and patient compliance, results in favorable clinical outcomes in a non‐surgical approach to the treatment (Brayer, Mellonig, Dunlap, Marinak, & Carson, 1989; Herrera, 2016). The effectiveness of SRP becomes limited by instrument limitations (Stambaugh, Dragoo, Smith, & Carasali, 1981), where curet efficiency decreases below 3.73‐mm pocket depth and reaches a limit of ~6‐mm depth. Beyond a critical probing depth of 5.4 mm, open flap debridement, a form of surgical periodontal therapy, may be preferred (Heitz‐Mayfield & Lang, 2013; Lindhe, Socransky, Nyman, Haffajee, & Westfelt, 1982).
The goals of periodontal surgical therapy are to treat periodontal disease or modify the morphological status of the periodontium, enabled by surgical access to deeper defects (Comprehensive Periodontal Therapy, 2011). Periodontal regeneration aims to facilitate the formation of new bone, cementum, and a functionally oriented PDL at a site deprived of its initial attachment apparatus (Giannobile & McClain, 2015). (Figure 3). Systematic reviews and consensus reports from the American Academy of Periodontology (AAP) are published in the Journal of Periodontology, including practical application reports. This review is not intended as a comprehensive clinical review. Still, it serves to highlight advances in tissue engineering specifically to the intersection of developmental biology and regeneration, and we encourage interested readers to consult the AAP.
FIGURE 3.
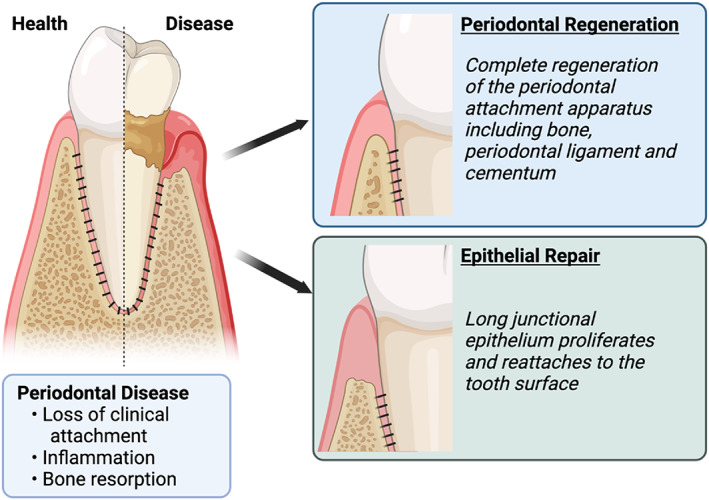
Therapeutic outcomes in the treatment of periodontal disease include periodontal regeneration and epithelial repair. Figure prepared with Biorender
Guided bone regeneration seeks to fulfill the specific goal of bone regeneration in preparation for dental implant placement without implicit concern for the PDL. These procedures may include alveolar ridge reconstruction, sinus floor augmentation, and resurrection of preimplant osseous defects. Guided tissue regeneration (GTR) seeks to reconstitute lost periodontal structures through different tissue responses, allowing for a renewal of the periodontal attachment apparatus, including bone, PDL, and cementum (Melcher, 1976; Wolff, 2000). Clinical indications of GTR are summarized by Wolff (Wolff, 2000). In addition to barrier membranes described above which allow for selective repopulation of the root surface, growth factors play a critical role in development and regeneration. They are a vital component of tissue engineering therapies.
In vitro studies of PDL‐SCs demonstrate their responsiveness to exogenous growth factor administration and secreted factors from neighboring cells found in the periodontium. Bone morphogenic proteins BMP‐2 and BMP‐7 and vascular endothelial growth factor increase the osteogenic capacity of PDL‐SCs (Hakki et al., 2014; Maegawa et al., 2007). Transforming growth factor beta (TGF‐β1) and connective tissue growth factor, and fibroblast growth factor (FGF) improve the fibroblastic differentiation (Fujii et al., 2010; Yuda et al., 2015). FGF‐2 promotes PDL‐SC proliferation but reverses the effects of BMP‐2 on osteogenic differentiation when co‐administered with J. H. Lee, Um, Jang, and Seo (2012). Their sequential administration may be optimized to encourage both PDL‐SC expansion then osteogenic differentiation. FGF‐2 administration to PDL‐SCs and MSCs upregulates the expression of BMP receptor‐1B (BMPR‐1B), enhancing the osteogenic effects of BMP‐2 (Nakamura et al., 2005). Growth factors may also play an essential role in maintaining the stemness of PDL‐SCs in treatment and long‐term in vitro culture.
Several growth factor therapies have received FDA approval in various indications of periodontal regeneration, and their clinical use has been validated in on‐label and off‐label uses. A systematic review from the 2015 AAP Regeneration Workshop highlights that biologics are generally comparable and compatible with allograft and GTR and superior to open flap debridement procedures for treating infrabony defects. These positive outcomes can be maintained over long periods, greater than 10 years (Kao, Nares, & Reynolds, 2015; Reynolds et al., 2015). Table 1 provides an overview of biologics currently used in clinical periodontal regeneration.
TABLE 1.
Growth factors used in clinical periodontal regeneration
| Biologic | Trade name | Source | FDA‐approved indications | Biologic significance | Mechanism | Clinical trials |
|---|---|---|---|---|---|---|
| Enamel matrix derivative | Emdogain (Straumann) | Swine tooth germ (dispersed in propylene glycol alginate matrix) containing enamel matrix proteins, largely amelogenins |
|
Enamel matrix proteins are secreted by Hertwig's epithelial root sheath during development of the enamel organ (Kornman, Giannobile, & Duff, 2017) | Recruitment of cementoblasts to form neocementum [154], fibroblasts [155], osteoblasts [156], and PDLSCs [157, 158] leading to mineralization. EMD remains on the root surface for up to 4 weeks [159] |
Infrabony defect [Miron et al., 2013, 160–162] Furcation defect [163, 164] Gingival recession [165–167] |
| Platelet derived growth factor | GEM‐21S (Lynch Biologics) | rh‐PDGF‐ββ |
Note: Approved for use in combination with beta‐tricalcium phosphate biomaterial |
PDGF is secreted by platelets upon activation and triggers fibroblast proliferation, migration, and differentiation [168, 169] |
Migration and proliferation of osteoblasts, PDL cells and cementoblasts. Stimulation of angiogenesis (Wikesjo, Sorensen, Kinoshita, Jian Li, & Wozney, 2004) |
Intrabony periodontal defect [94, 170, 171] Furcation defects [170, 172, 173] Soft tissue augmentation [174, 175] |
| Bone Morphogenic protein | Infuse (Medtronic) | rh‐BMP2 |
Note: Approved for use in combination with carrier/scaffold such as adsorbable collagen sponge |
BMPs play crucial roles in all organ systems. Endogenous BMP signaling is tightly regulated and involved in the skeletal development [176, 177] |
BMP‐2 stimulates osteoblast chemotaxis, differentiation, alkaline phosphatase activity, and osteocalcin synthesis/mineralization [178, 179] |
Sinus augmentation: [180–182] Alveolar ridge + socket preservation: [183, 184] |
| Platelet rich plasma (PRP) | N/A | Autologous blood with concentrated platelets >250,000 platelets/μl | The FDA does not regulate or approve of PRP treatments; the kits and devices used to prepare PRP require clearance [185] | Circulating growth factors and other inductive molecules to promote healing are concentrated and used autogenously | The therapeutic effects of PRP are attributed to “bioactive factors” [186, 187]. A discrete mechanism of action is not defined | A comprehensive review of PRP in periodontal regeneration of intrabony defects is summarized by Rosello‐Camps et al. [188] |
The summary in Table 1 demonstrates significant interest and development in growth factor therapeutics used in periodontal regeneration. These developments are informed by decades of investigational research into inductive factors which play pivotal roles in periodontal and dentoalveolar development and maintenance. Significant progress has been made in their routine clinical implementation, but not without limitations:
Enamel matrix derivative (EMD): Initial studies suggested up to three times greater defect fill when compared to open flap debridement alone (Heijl, Heden, Svardstrom, & Ostgren, 1997) and significant clinical attachment gain (Miron et al., 2016). Other trials have failed to demonstrate heterogeneity in its clinical effect (Esposito, Grusovin, Papanikolaou, Coulthard, & Worthington, 2009; Miron et al., 2013) or lack of effect compared to non‐biologic GTR protocols in single intrabony defects (Gutierrez, Mellonig, & Cochran, 2003; Mombelli, Brochut, Plagnat, Casagni, & Giannopoulou, 2005). Various studies have demonstrated heterogeneity in regenerative outcomes due to adsorption and efficient delivery of EMD. A new commercial product, Osteogain (Straumann), is in development which aims to improve the combination of EMD and bone grafting materials (J. Chen et al., 2015; Miron et al., 2012).
Platelet‐derived growth factor (PDGF): rhPDGF‐ββ combined with a bioresorbable material, beta‐tricalcium phosphate, enabled its delivery and was the first entirely synthetic product approved by the FDA to treat periodontal‐related defects (Nevins et al., 2005). Tavelli et al. (Wikesjo et al., 2004) provides an in‐depth review of its clinical potential, which suggests strong evidence for its efficacy in regenerating infrabony defects when used in conjunction with a bone matrix. Various clinical and human histologic studies have demonstrated evidence of new bone, PDL, and cementum formation following rhPDGF‐ββ administration.
In addition to growth factors, significant progress in biomaterials engineering for periodontal regeneration has been made and adopted into widespread clinical practice. Given that patient behaviors appreciably influence clinical outcomes and surgical approach rather than tooth and defect characteristics taking advantage of the inductive features of biomaterials may significantly enhance regenerative effects (Cochran et al., 2015; Kao et al., 2015; Reynolds et al., 2015). The surgical technique also undoubtedly plays a role in their clinical success.
A significant reason for using biomaterials is to facilitate the delivery of biologic agents to the defect site. These materials simultaneously provide an artificial matrix to accelerate wound healing, repair, and regeneration (Swanson & Ma, 2020). Biomaterials can be further engineered to enable delivery with sensitivity to spatial and temporal requirements of biologic agents and healing processes. Somerman (2010) describes that while agents on the market (Gem21S, Emdogain, FGF‐2) have demonstrated promise in various aspects of periodontal regeneration, there is room for improvement in the predictability of their ability to achieve sufficient regeneration, which may require combinations of growth factors with controlled deliveries. Herein we will discuss recent advances in growth factors, biomaterial delivery mechanisms, and areas for continued investigation.
5. DEVELOPMENT OF NEXT‐GENERATION REGENERATIVE THERAPEUTICS
A literature review illustrates significant progress in periodontal regeneration in the last 30 years, and gaps for improvement have been identified. Regeneration of the PDL requires the concerted, synchronized activity of multiple cell types and molecular processes. We believe that an intimate understanding of the development and physiologic maintenance of the PDL and surrounding structures is a prerequisite to developing next‐generation regenerative protocols and technologies. The ideal regenerative therapeutic will satisfy the following regenerative criteria:
cementoblastogenesis on the tooth root surface;
oblique insertion of PDL fibers into cementum and alveolar bone;
vital supporting bone.
We believe the key challenges in achieving these regenerative goals are the management of vascularization in periodontal tissues, the potential for microbial contamination into the defect, and masticatory forces present during healing.
5.1. Next‐generation growth factors for periodontal regeneration
Next‐generation growth factor therapeutics must consider this complexity of the periodontium and will draw inspiration from an improved understanding of its physiologic development. We will highlight advances in growth factor therapeutics that have demonstrated promising in vitro and preclinical in vivo results, warranting further development.
BMP‐6: BMP‐6 is similar in structure to BMP 5 and 7. It is responsible for osteoblast differentiation. In a study of BMP‐6 and collagen sponge carriers applied to periodontal fenestration defects in rats, complete osseous healing occurred in BMP‐6‐treated animals after 4‐weeks (K. K. Huang, Shen, Chiang, Hsieh, & Fu, 2005).
GDF‐7: In a pilot study, the potential of growth and differentiation factor‐7 (GDF‐7)/BMP‐12 to stimulate PDL formation was evaluated in a supra‐alveolar periodontal defect model. This study suggested that GDF‐7 has a significant potential to support the regeneration of the PDL (Wikesjo et al., 2004).
GDF‐5: GDF‐5 (BMP‐14) is another member of the TGF‐β superfamily of interest for the periodontal regeneration (Moore, Dickinson, & Wikesjö, 2010). BMP‐14 is expressed in developing periodontal tissues (Morotome, Goseki‐Sone, Ishikawa, & Oida, 1998) and primordial cartilage in early limb development (Francis‐West et al., 1999; Storm & Kingsley, 1999). GDF‐5 has a high binding affinity to BMPR1B, BMPR2 (Nishitoh et al., 1996), and Activin Type II receptors (Klammert et al., 2015). Human clinical trials have demonstrated its efficacy in periodontal regeneration: intrabony defect (Stavropoulos, Windisch, et al., 2011) and sinus augmentation (Koch, Becker, Terheyden, Capsius, & Wagner, 2010; Stavropoulos, Becker, et al., 2011). GDF‐5 was under development by Scil Technology, GmbH, as MD05. Scil Technology, GmbH is no longer pursuing clinical development, and the asset was licensed to Medtronic for development (Emerton et al., 2011). Recent evidence in protein engineering suggests the GDF‐5 mutant BB‐1 may have enhanced osteoinductive capacity in a large animal model (Gunnella et al., 2018).
Teriparatide (Forteo, Eli Lilly, Inc.): Teriparatide is a synthetic form of human parathyroid hormone (PTH), which stimulates new bone formation. In the first human clinical trial with teriparatide in periodontal healing, 40 patients with periodontitis were treated with teriparatide or placebo once daily for 6 weeks following open flap debridement. Treatment with teriparatide led to a greater resolution in osseous defects and improved clinical attachment, where even short dosing of the drug had significant long‐term effects (Bashutski et al., 2010; Grover, Luthra, & Maroo, 2013).
FGF‐2: FGF‐2 promotes the proliferation of osteoblasts and fibroblasts (Murakami, 2011). FGF‐2 also possesses angiogenic and mitogenic activity on mesenchymal cells within the PDL (Suarez‐Lopez Del Amo, Monje, Padial‐Molina, Tang, & Wang, 2015). While not FDA‐approved for its use in the US, FGF‐2 is approved for human clinical use in Japan in a topical formulation for regenerating periodontal tissues destroyed by periodontitis Kitamura et al. (2011) (Kaken Pharmaceuticals). It demonstrates significant regenerative potential compared to vehicle treatment but has not been well‐studied in head‐to‐head comparisons with other growth factors (Cochran et al., 2016).
Exosome therapy: Exosomes are a subset of extracellular vesicles, lipid‐bound nanoparticles secreted by cells and containing various signaling molecules, including DNAs, RNAs, and proteins (Narayanan, Huang, & Ravindran, 2016; Swanson & Mishina, 2022). Exosomes have been thought to be nature's endogenous nanoparticle delivery platform, and in particular, their miRNA cargo and growth factor are of significant interest (Swanson, Gong, et al., 2020; Swanson & Mishina, 2022; Swanson, Zhang, et al., 2020). Recently, human bone marrow stromal cell‐derived exosomes were demonstrated as a promising therapeutic in a preclinical rat periodontitis model to reduce tissue destruction and immune cell infiltration (Yue et al., 2022). The authors identified enrichment miRNAs associated with negative regulation of inflammatory response and increased protein abundance of factors including FGF‐6, insulin growth factor (IGF‐1), and interleukins: IL‐1ra, IL‐16, and IL‐3 concentrated in exosomes.
5.2. Next‐generation signal delivery for periodontal regeneration
Small molecule drugs and protein cargo can be encapsulated by biodegradable materials through double emulsion technologies yielding bioactive molecules encapsulated within a biodegradable polymeric shell. Biodegradable biomaterials, which degrade over time in the body, are adequate to allow for the sustained release of drugs and inductive factors. As the material degrades due to hydrolysis in the physiologic environment, factors escape entrapment and are exposed within the defect site (Langer & Vacanti, 2016; Ma, 2008). PDGF‐ββ (Wei, Jin, Giannobile, & Ma, 2006) has been demonstrated to tolerate encapsulation in a poly(glycolic‐co‐lactic acid) PLGA polymeric vector, maintaining their biologic efficacy following encapsulation and release. PDGF‐ββ encapsulated in microspheres is easily attached to nanofibrous tissue engineering scaffolds (Wei et al., 2006). Parathyroid hormone and exosomes each represent examples of biologics requiring suitable delivery platforms to enable their delivery. Parathyroid hormone (Teriparatide) requires a pulsatile administration to induce bone formation, given the observation that continuous PTH exposure results in bone resorption. Dang and others developed an innovative mechanism for the preprogrammed long‐term pulsatile delivery of PTH, allowing for anabolic bone regeneration (Dang, Koh, Danciu, McCauley, & Ma, 2017; Dang, Koh, Jin, McCauley, & Ma, 2017; X. Liu, Pettway, McCauley, & Ma, 2007). Lipid nanovesicle exosomes are much larger than protein biologics and require stabilization of their lipid membrane, internal protein, and nucleic acid cargo. Swanson and colleagues demonstrated the first controlled release system for the efficient encapsulation and controlled release of exosomes and extracellular vesicles in a nanoparticle system, allowing for their sustained release (Swanson, Gong, et al., 2020; Swanson, Zhang, et al., 2020). The release kinetics of encapsulated exosomes is tunable based on the biomaterial composition.
To regenerate hierarchically structured tissues, such as periodontium, it is essential to trigger appropriate and precise signals to direct cell populations to form appropriate tissue types in the correct anatomical locations (Gonzalez‐Fernandez et al., 2019). The specific spatial or temporal delivery of signaling molecules, such as growth factors, can be challenging. To overcome those limitations, various gene therapies have been developed specifically for periodontal diseases, allowing for sustained synthesis and secretion of one or multiple growth factors by genetically modifying cells (Figure 4) (Galli et al., 2021).
FIGURE 4.
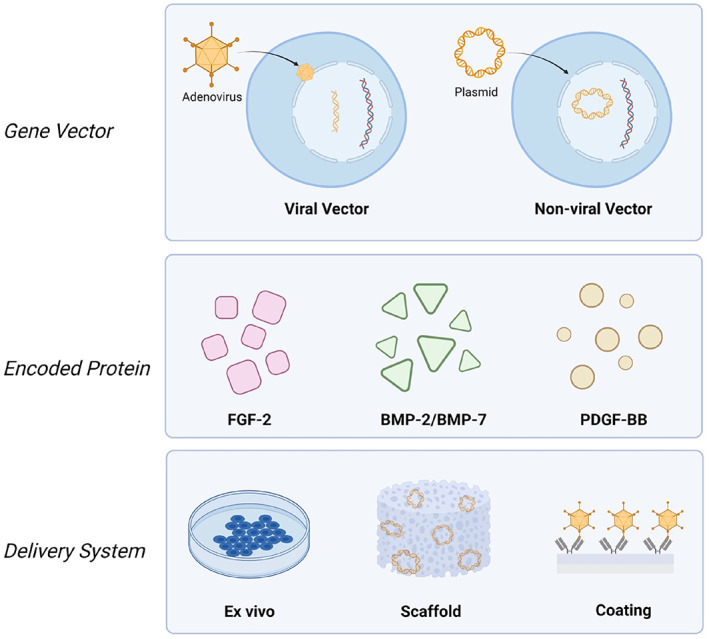
Schematic representation of gene delivery technologies and their various embodiments which may be used in periodontal regeneration. Figure made with Biorender
Viral vectors used for gene delivery include adenovirus (Ad), adeno‐associated virus, lentivirus, retrovirus, and baculovirus, each of which has its advantages and disadvantages (Galli et al., 2021). Ad has been frequently used in periodontal regeneration because of several unique features compared with other viral vectors: (1) high transfection efficiency in both dividing and non‐dividing cells; (2) does not induce apparent phenotypic changes; and (3) does not integrate into host genome (Gu et al., 2004). Dunn et al. delivered Ad‐BMP‐7 using a collagen matrix around titanium dental implants to treat peri‐implant osseous defects and revealed the sustained transgene expression for up to 10 days at the osteotomy sites (Dunn et al., 2005). In addition to Ad‐BMP‐7, Ad‐PDGF‐B used in large periodontal osseous defects has been demonstrated safe with improved alveolar bone and cementum regeneration for possible use in human clinical studies (Chang et al., 2009; Jin, Anusaksathien, Webb, Printz, & Giannobile, 2004).
Based on the results from previous studies and the architecture of periodontium consisting of PDL fibroblasts and osteoblasts, various groups have developed localized and sustained dual‐gene vector delivery systems to treat periodontal defects. Hao et al. (2016) developed a chemical vapor deposition‐based dual‐gene delivery system, which has been demonstrated to successfully deliver Ad‐BMP‐7 and Ad‐PDGF‐B to human PDL cells, resulting in highly compartmentalized and sustained protein production compared with physical absorption. In another study, a polymeric scaffold containing Ad‐BMP‐7 with Ad‐PDGF‐B was implanted in a preclinical model of a buccal dehiscence defect, concluding that either Ad‐BMP‐7 or Ad‐PDGF‐B was capable of promoting periodontal regeneration individually. Still, their combination synergistically enabled the wound healing (Y. Zhang et al., 2015).
Although the efficiency of transfecting cells is relatively high with viral vectors, concerns surrounding immunogenicity and cytotoxicity must be acknowledged (Ramamoorth & Narvekar, 2015). Non‐viral vectors such as plasmids, which are small circular DNA structures that can replicate in the cell independently of chromosomes, have gained significant traction due to their reduced pathogenicity, low cost, and ease of production (L. Jiang et al., 2020). Although the development of gene therapy in periodontal regeneration is at the early stage and warrants additional investigation, it has demonstrated outstanding potential for catalyzing coordinated regeneration of both soft and hard tissues in the periodontium (Woo, Cho, Tarafder, & Lee, 2021).
5.3. Next‐generation biomaterial design for periodontal regeneration
The physical design of biomaterial scaffolds relies on physical cues to induce cell and tissue fate in regeneration, for example, texture and porosity (Swanson & Ma, 2020). Nanofibers enable increased cell adhesion to the biomaterial and an increased surface area for the adsorption of extracellular matrix proteins (R. Zhang & Ma, 2000). Porosity allows tissue integration, cell infiltration, and nutrient/waste exchange. The specific size of pores within biomaterials has been recently correlated to driving distinct tissue fates by modulating construct vascularization, extracellular matrix composition, and gene expression (Gupte et al., 2018; Swanson et al., 2022, 2021). Based on these understandings, various design motifs may be combined in spatially distinct regions to create composite scaffolds capable of regenerating tissues and their functional interfaces within complex microenvironments.
Multiphasic scaffolds may be designed to engineer the PDL and its interfaces based on architectural and biochemical composition variations throughout a construct (Jeon, Vaquette, Klein, & Hutmacher, 2014). Various examples of multiphasic scaffolds are described in Table 2. Considering the hierarchical structure of the periodontium, Ivanovski et al. summarized critical aspects of multiphasic scaffold design for periodontal tissue engineering: (a) compartmentalization of bone and PDL; (b) promotion of cementum formation on the root surface; and (c) formation of appropriately oriented PDL fibers (Ivanovski, Vaquette, Gronthos, Hutmacher, & Bartold, 2014). According to these general principles, either biphasic (bone‐PDL or PDL‐cementum) or triphasic scaffolds (bone‐PDL‐cementum) were designed previously for the integrated periodontium regeneration (Woo et al., 2021; Yao et al., 2022).
TABLE 2.
Design and fabrication of multiphasic scaffolds for periodontal tissue engineering
| CM compartment | PDL compartment | AB compartment | Main outcomes | References |
|---|---|---|---|---|
| Biphasic | ||||
| NA | 3D‐waxing‐printed PGA scaffold | 3D‐waxing‐printed PCL scaffold |
Parallel‐ and obliquely‐oriented PDL fibers within the construct |
Park et al. 2010 [189] |
| NA |
Solution electrospun membrane |
FDM scaffold | Cementum‐like tissue deposition at the dentin‐cell sheets interface |
Vaquette et al. 2012 (Sowmya et al., 2017) |
| NA |
Solution electrospun membrane |
Melt electrospun scaffold | Tissue integration between the bone and PDL |
Vaquette et al. 2019 [190] |
| Triphasic | ||||
|
100 μm microchannel + human amelogenins |
600‐μm microchannel + CTGF |
300‐μm microchannel + BMP‐2 |
Formation of bone, PDL, and cementum/dentin‐like tissue in the various scaffold compartments with characteristic histologic features |
Lee et al. 2014 (Van Steenberghe, 2000) |
|
Chitin‐PLGA/nBGC nanocomposite + CEMP1 |
Chitin‐PLGA hydrogel + FGF‐2 |
Chitin‐PLGA/nBGC nanocomposite + PRP |
Defect closure; cementum, PDL, and alveolar bone formation | Sowmya et al., 2017 (Gault et al., 2010) |
Abbreviations: AB, alveolar bone: CM, cementum; PDL, periodontal ligament.
Vaquette et al. (2012) reported a biphasic tissue‐engineered construct for periodontal regeneration with a porous PDL compartment and stiff bone compartment. Excellent tissue integration between bone and PDL compartments and the tooth root interface with the establishment of Sharpey's fibers was observed. 3D‐printing techniques may also be employed in this area, as demonstrated by Park et al. (2012) in a PDL‐bone composite scaffold. An ectopic periodontal regeneration model showed a significantly more organized fibrous connective tissue with calcified tissue layers on the dentin surface in biphasic scaffolds compared to a random‐porous structure. Triphasic scaffolds have the potential for complete regeneration of the periodontium to promote the generation of two mineralized tissues and one soft tissue in the middle. In 2014, C. H. Lee et al. (2014) printed seamless scaffolds with tissue‐specific microstructures consisting of three phases, yielding aligned PDL‐like collagen fibers inserted into bone‐like tissue and putative cementum matrix protein‐positive tissues. Sowmya et al. (2017) suggested triphasic nanocomposite hydrogel scaffolds combined with three tissue‐specific growth factors (CEMP‐1 for the cementum layer, FGF‐2 for the PDL layer, and PDGF for the bone layer). The results confirmed the formation of new cementum, fibrous PDL, and alveolar bone with well‐defined bony trabeculae in a rabbit periodontal defect (Sowmya et al., 2017).
Although multiphasic scaffold strategies seem to be well‐suited for periodontal tissue engineering and regeneration in terms of their ability to stimulate coordinated responses in both soft and hard tissues, there is a scarcity of work and apparent limitations. First, as most of the multiphasic constructs were made solely from polycaprolactone, the biomaterial is hydrophobic and does not provide any specific biochemical cues. For non‐3D‐printed scaffolds, the thickness and shape of the constructs were not highly adjustable, preventing a precise match between the dimensions of the defect. As of 2022, no clinical studies have been published in the literature. Designing a multiphasic scaffold with strong cohesion between different phases, sufficient surgical handling properties, and the ability to customize the morphology of the scaffold to adapt to clinical defects of varying shapes and sizes are key points to be considered for future clinical translation (Galli et al., 2021).
In addition to the general principle of biomaterial design for periodontal regeneration, which aims for coordinated regeneration of PDL, cementum, and alveolar bone, a key question in the field of periodontal regeneration is whether a PDL tissue is advantageous for dental implants, as opposed to implant osseointegration (Giannobile, 2010). In one way, implementing hybrid biomaterials, which can promote the formation of implant‐ligament interfaces, offers tremendous potential for oral implants to maintain form, function, and proprioceptive responses more similar to a natural tooth (Van Steenberghe, 2000). On the other hand, technologies demonstrate significant unpredictability in human clinical trials and raise concerns around cost and the impractical application of cell‐based tissue engineering technologies (Gault et al., 2010).
5.4. The use and need for stem cells in periodontal regeneration
A key consideration in regenerative therapeutics for periodontal regeneration is the role of stem cells. Classical tissue engineering paradigms involve transplanting exogenous stem cell sources into a defect site (Langer & Vacanti, 1993). Recently, various reports have demonstrated that cell transplantation does not directly accelerate wound healing in craniofacial defects (Kitami et al., 2016). Concerning the PDL, Yan et al. (2015) showed similar regenerative results from a hydrogel loaded with PDL‐SCs and an empty hydrogel after 4 weeks of wound healing in a rat infrabony defect and concluded that the contribution of hydrogel‐incorporated cells to periodontal regeneration could not be ascertained. Chemotactic growth factors and extracellular vesicles, which recruit endogenous cells to a defect site, may be sufficient to catalyze the regeneration (Gegout et al., 2021). Chew et al. demonstrated that mesenchymal stem cell‐derived exosomes, loaded in a collagen sponge, catalyzed periodontal regeneration in a rat model by activating pro‐survival AKT and ERK signaling (Chew et al., 2019). Yue et al. similarly demonstrated that weekly exosome injections into gingival tissues suppressed pathogen‐triggered inflammatory responses by macrophages and showed their ability to be used as the most modulation agent in the management of periodontitis (Yue et al., 2022). Experiments studying the role of conditioned media and various secreted factors in the periodontium will undoubtedly lead to discoveries of bioactive molecules involved in physiologic tissue development, maintenance, and repair (Lin et al., 2021). In this way, taking advantage of endogenous cells rather than relying on the transplantation of exogenous stem cells represents a significantly decreased regulatory and cost burden for the clinical translation of these exciting new technologies. These findings highlight the importance of understanding the molecular signatures and character of the periodontal stem cell population and its neighboring stem cell populations (Pagella, de Vargas Roditi, Stadlinger, Moor, & Mitsiadis, 2021).
6. CHALLENGES AND FUTURE DEVELOPMENTS
Biomaterials and growth factors, and their synergies together, represent significant technologic advantages for the future of precision periodontal medicine and the predictable periodontal regeneration (Kornman et al., 2017). Growth factors can induce cell migration, tissue morphogenesis, phenotype induction, vascularization, and healing. Biomaterials provide a matrix for regeneration and facilitate the spatial and temporal controlled release of inductive substances to catalyze regeneration. An intimate understanding of the developmental biology underlying the periodontium is critical for designing and identifying promising interventional strategies which can improve patient outcomes toward complete regeneration of the periodontal structures, including cementum, PDL, and alveolar bone. This encouraging progress in these areas advances clinical translation and the use of multiple biologic therapeutics for periodontal regenerative medicine. Further development is needed to improve the predictability of these outcomes, particularly considering the long‐term stability of regenerated periodontal tissues. Periodontal regeneration has been a rapidly growing field with significant excitement since its inception, with a tremendous potential to advance periodontal healthcare.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (R01DE027662 to Yuji Mishina and F30DE029359 to W. Benton Swanson). The authors would like to acknowledge Dr William V. Giannobile, DMD, DMSc (Department of Oral Medicine, Infection, and Immunity, Harvard School of Dental Medicine, Boston, MA, USA) for his thorough review and contribution to strengthening this work.
Swanson, W. B. , Yao, Y. , & Mishina, Y. (2022). Novel approaches for periodontal tissue engineering. genesis, 60(8‐9), e23499. 10.1002/dvg.23499
Funding information National Institute of Dental and Craniofacial Research, Grant/Award Numbers: F30DE029359, R01DE027662
DATA AVAILABILITY STATEMENT
All data related to this manuscript are provided in relevant citations and included in the manuscript.
REFERENCES
- Aiyegbusi, O. L. , Macpherson, K. , Elston, L. , Myles, S. , Washington, J. , Sungum, N. , … Calvert, M. J. (2020). Patient and public perspectives on cell and gene therapies: A systematic review. Nature Communications, 11(1), 6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albandar, J. M. , Susin, C. , & Hughes, F. J. (2018). Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. Journal of Periodontology, 89, S183–S203. [DOI] [PubMed] [Google Scholar]
- Bashutski, J. D. , Eber, R. M. , Kinney, J. S. , Benavides, E. , Maitra, S. , Braun, T. M. , … McCauley, L. K. (2010). Teriparatide and osseous regeneration in the oral cavity. New England Journal of Medicine, 363(25), 2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho, J. , Machado, V. , Leira, Y. , Proenca, L. , Chambrone, L. , & Mendes, J. J. (2022). Economic burden of periodontitis in the United States and Europe: An updated estimation. Journal of Periodontology, 93(3), 373–379. [DOI] [PubMed] [Google Scholar]
- Brayer, W. K. , Mellonig, J. T. , Dunlap, R. M. , Marinak, K. W. , & Carson, R. E. (1989). Scaling and root planing effectiveness: The effect of root surface access and operator experience. Journal of Periodontology, 60(1), 67–72. [DOI] [PubMed] [Google Scholar]
- Burdick, J. A. , Mauck, R. L. , Gorman, J. H. , & Gorman, R. C. (2013). Acellular biomaterials: An evolving alternative to cell‐based therapies. Science Translational Medicine, 5(176), 176ps4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser, D. , Sennerby, L. , & De Bruyn, H. (2000). Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000, 73(1), 7–21. [DOI] [PubMed] [Google Scholar]
- y Baena, A. R. , Casasco, A. , & Monti, M. (2022). Hypes and hopes of stem cell therapies in dentistry: A review. Stem Cell Reviews and Reports, 18, 1294–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, B. P. , & Leong, K. W. (2008). Scaffolding in tissue engineering: General approaches and tissue‐specific considerations. European Spine Journal, 17(Suppl 4), 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, P.‐C. , Cirelli, J. A. , Jin, Q. , Seol, Y.‐J. , Sugai, J. V. , D'Silva, N. J. , … Giannobile, W. V. (2009). Adenovirus encoding human platelet‐derived growth factor‐B delivered to alveolar bone defects exhibits safety and biodistribution profiles favorable for clinical use. Human Gene Therapy, 20(5), 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F.‐M. , Gao, L.‐N. , Tian, B.‐M. , Zhang, X.‐Y. , Zhang, Y.‐J. , Dong, G.‐Y. , … Jin, Y. (2016). Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Research & Therapy, 7(1), 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Li, X. , Su, Y. , Zhang, D. , Wen, X. , Nie, X. , … Deng, M. (2015). A micro‐computed tomography study of the relationship between radicular grooves and root canal morphology in mandibular first premolars. Clinical Oral Investigations, 19(2), 329–334. [DOI] [PubMed] [Google Scholar]
- Chew, J. R. J. , Chuah, S. J. , Teo, K. Y. W. , Zhang, S. , Lai, R. C. , Fu, J. H. , … Toh, W. S. (2019). Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomaterialia, 89, 252–264. [DOI] [PubMed] [Google Scholar]
- Chiego, D. J. (2018). Essentials of oral histology and embryology: A clinical approach (5th ed.). St. Louis, MO: Elsevier. [Google Scholar]
- Cochran, D. L. , Cobb, C. M. , Bashutski, J. D. , Chun, Y.‐H. P. , Lin, Z. , Mandelaris, G. A. , … Rios, H. F. (2015). Emerging regenerative approaches for periodontal reconstruction: A consensus report from the AAP regeneration workshop. Journal of Periodontology, 86(2‐s), S153–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran, D. L. , Oh, T. J. , Mills, M. P. , Clem, D. S. , McClain, P. K. , Schallhorn, R. A. , … Takemura, A. (2016). A randomized clinical trial evaluating rh‐FGF‐2/β‐TCP in periodontal defects. Journal of Dental Research, 95(5), 523–530. [DOI] [PubMed] [Google Scholar]
- Comprehensive Periodontal Therapy . (2011). A statement by the American Academy of periodontology. Journal of Periodontology, 82(7), 943–949. [DOI] [PubMed] [Google Scholar]
- Dang, M. , Koh, A. J. , Danciu, T. , McCauley, L. K. , & Ma, P. X. (2017). Preprogrammed long‐term systemic pulsatile delivery of parathyroid hormone to strengthen bone. Advanced Healthcare Materials, 6(3), 1600901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, M. , Koh, A. J. , Jin, X. , McCauley, L. K. , & Ma, P. X. (2017). Local pulsatile PTH delivery regenerates bone defects via enhanced bone remodeling in a cell‐free scaffold. Biomaterials, 114, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, C. A. , Jin, Q. , Taba, M., Jr. , Franceschi, R. T. , Rutherford, R. B. , & Giannobile, W. V. (2005). BMP gene delivery for alveolar bone engineering at dental implant defects. Molecular Therapy, 11(2), 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Gezawi, M. , Wölfle, U. C. , Haridy, R. , Fliefel, R. , & Kaisarly, D. (2019). Remineralization, regeneration, and repair of natural tooth structure: Influences on the future of restorative dentistry practice. ACS Biomaterials Science & Engineering, 5(10), 4899–4919. [DOI] [PubMed] [Google Scholar]
- Emerton, K. B. , Drapeau, S. J. , Prasad, H. , Rohrer, M. , Roffe, P. , Hopper, K. , … Cochran, D. L. (2011). Regeneration of periodontal tissues in non‐human primates with rhGDF‐5 and beta‐tricalcium phosphate. Journal of Dental Research, 90(12), 1416–1421. [DOI] [PubMed] [Google Scholar]
- Esposito, M. , Grusovin, M. G. , Papanikolaou, N. , Coulthard, P. , & Worthington, H. V. (2009). Enamel matrix derivative (Emdogain®) for periodontal tissue regeneration in intrabony defects. Cochrane Database of Systematic Reviews, 2, CD003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts, V. , van der Zee, E. , Creemers, L. , & Beertsen, W. (1996). Phagocytosis and intracellular digestion of collagen, its role in turnover and remodelling. The Histochemical Journal, 28(4), 229–245. [DOI] [PubMed] [Google Scholar]
- Feng, F. , Akiyama, K. , Liu, Y. , Yamaza, T. , Wang, T. M. , Chen, J. H. , … Shi, S. (2010). Utility of PDL progenitors for in vivo tissue regeneration: A report of 3 cases. Oral Diseases, 16(1), 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis‐West, P. H. , Abdelfattah, A. , Chen, P. , Allen, C. , Parish, J. , Ladher, R. , … Archer, C. W. (1999). Mechanisms of GDF‐5 action during skeletal development. Development, 126(6), 1305–1315. [DOI] [PubMed] [Google Scholar]
- Freeman, E. , & ten Cate, A. R. (1971). Development of the periodontium: An electron microscopic study. Journal of Periodontology, 42(7), 387–395. [DOI] [PubMed] [Google Scholar]
- Fujii, S. , Maeda, H. , Tomokiyo, A. , Monnouchi, S. , Hori, K. , Wada, N. , & Akamine, A. (2010). Effects of TGF‐β1 on the proliferation and differentiation of human periodontal ligament cells and a human periodontal ligament stem/progenitor cell line. Cell and Tissue Research, 342(2), 233–242. [DOI] [PubMed] [Google Scholar]
- Galli, M. , Yao, Y. , Giannobile, W. V. , & Wang, H.‐L. (2021). Current and future trends in periodontal tissue engineering and bone regeneration. Plastic and Aesthetic Research, 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, X. , Shen, Z. , Guan, M. , Huang, Q. , Chen, L. , Qin, W. , … Lin, Z. (2018). Immunomodulatory role of stem cells from human exfoliated deciduous teeth on periodontal regeneration. Tissue Engineering Part A, 24(17–18), 1341–1353. [DOI] [PubMed] [Google Scholar]
- Gault, P. , Black, A. , Romette, J. L. , Fuente, F. , Schroeder, K. , Thillou, F. , … Wurtz, T. (2010). Tissue‐engineered ligament: Implant constructs for tooth replacement. Journal of Clinical Periodontology, 37(8), 750–758. [DOI] [PubMed] [Google Scholar]
- Gegout, P. Y. , Stutz, C. , Olson, J. , Batool, F. , Petit, C. , Tenenbaum, H. , … Huck, O. (2021). Interests of exosomes in bone and periodontal regeneration: A systematic review. Advances in Experimental Medicine and Biology, 1341, 67–87. [DOI] [PubMed] [Google Scholar]
- Giannobile, W. V. (2010). Getting to the root of dental implant tissue engineering. Journal of Clinical Periodontology, 37(8), 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannobile, W. V. , & McClain, P. K. (2015). Enhancing periodontal health through regenerative approaches. Journal of Periodontology, 86(2‐s), S1–S3. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Fernandez, T. , Rathan, S. , Hobbs, C. , Pitacco, P. , Freeman, F. , Cunniffe, G. , … O'Brien, F. (2019). Pore‐forming bioinks to enable spatio‐temporally defined gene delivery in bioprinted tissues. Journal of Controlled Release, 301, 13–27. [DOI] [PubMed] [Google Scholar]
- Groeneveld, M. C. , Everts, V. , & Beertsen, W. (1993). A quantitative enzyme histochemical analysis of the distribution of alkaline phosphatase activity in the periodontal ligament of the rat incisor. Journal of Dental Research, 72(9), 1344–1350. [DOI] [PubMed] [Google Scholar]
- Grover, H. S. , Luthra, S. , & Maroo, S. (2013). Teriparatide: A novel means to ultimately achieve true regeneration!!! Journal of Clinical and Diagnostic Research, 7(8), 1820–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnella, F. , Kunisch, E. , Maenz, S. , Horbert, V. , Xin, L. , Mika, J. , … Bungartz, M. (2018). The GDF5 mutant BB‐1 enhances the bone formation induced by an injectable, poly(l‐lactide‐co‐glycolide) acid (PLGA) fiber‐reinforced, brushite‐forming cement in a sheep defect model of lumbar osteopenia. The Spine Journal, 18(2), 357–369. [DOI] [PubMed] [Google Scholar]
- Guo, W. , Chen, L. , Gong, K. , Ding, B. , Duan, Y. , & Jin, Y. (2012). Heterogeneous dental follicle cells and the regeneration of complex periodontal tissues. Tissue Engineering Part A, 18(5–6), 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte, M. J. , Swanson, W. B. , Hu, J. , Jin, X. , Ma, H. , Zhang, Z. , … Ma, P. X. (2018). Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomaterialia, 82, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, M. A. , Mellonig, J. T. , & Cochran, D. L. (2003). Evaluation of enamel matrix derivative as an adjunct to non‐surgical periodontal therapy. Journal of Clinical Periodontology, 30(8), 739–745. [DOI] [PubMed] [Google Scholar]
- Hakki, S. S. , Bozkurt, B. , Hakki, E. E. , Kayis, S. A. , Turac, G. , Yilmaz, I. , & Karaoz, E. (2014). Bone morphogenetic protein‐2, ‐6, and ‐7 differently regulate osteogenic differentiation of human periodontal ligament stem cells. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 102(1), 119–130. [DOI] [PubMed] [Google Scholar]
- Hao, J. , Cheng, K. C. , Kruger, L. G. , Larsson, L. , Sugai, J. V. , Lahann, J. , & Giannobile, W. V. (2016). Multigrowth factor delivery via immobilization of gene therapy vectors. Advanced Materials, 28(16), 3145–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijl, L. , Heden, G. , Svardstrom, G. , & Ostgren, A. (1997). Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. Journal of Clinical Periodontology, 24(9 Pt 2), 705–714. [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. , & Lang, N. P. (2013). Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontology 2000, 62(1), 218–231. [DOI] [PubMed] [Google Scholar]
- Herrera, D. (2016). Scaling and root planning is recommended in the nonsurgical treatment of chronic periodontitis. The Journal of Evidence‐Based Dental Practice, 16(1), 56–58. [DOI] [PubMed] [Google Scholar]
- Hoffman, T. , Khademhosseini, A. , & Langer, R. (2019). Chasing the paradigm: Clinical translation of 25 years of tissue engineering. Tissue Engineering Part A, 25(9–10), 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, K. K. , Shen, C. , Chiang, C. Y. , Hsieh, Y. D. , & Fu, E. (2005). Effects of bone morphogenetic protein‐6 on periodontal wound healing in a fenestration defect of rats. Journal of Periodontal Research, 40(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Corpas, L. S. , Martens, W. , Jacobs, R. , & Lambrichts, I. (2011). Histomorphological study of myelinated nerve fibres in the periodontal ligament of human canine. Acta Odontologica Scandinavica, 69(5), 279–286. [DOI] [PubMed] [Google Scholar]
- Ivanovski, S. , Vaquette, C. , Gronthos, S. , Hutmacher, D. , & Bartold, P. (2014). Multiphasic scaffolds for periodontal tissue engineering. Journal of Dental Research, 93(12), 1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, J. E. , Vaquette, C. , Klein, T. J. , & Hutmacher, D. W. (2014). Perspectives in multiphasic osteochondral tissue engineering. The Anatomical Record, 297(1), 26–35. [DOI] [PubMed] [Google Scholar]
- Jiang, L. , Ding, Z. , Xia, S. , Liu, Y. , Lei, S. , Zhong, M. , & Chen, X. (2020). Poly lactic‐co‐glycolic acid scaffold loaded with plasmid DNA encoding fibroblast growth factor‐2 promotes periodontal ligament regeneration of replanted teeth. Journal of Periodontal Research, 55(4), 488–495. [DOI] [PubMed] [Google Scholar]
- Jiang, N. , Guo, W. , Chen, M. , Zheng, Y. , Zhou, J. , Kim, S. G. , … Mao, J. J. (2016). Periodontal ligament and alveolar bone in health and adaptation: Tooth movement. Frontiers of Oral Biology, 18, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Q. , Anusaksathien, O. , Webb, S. A. , Printz, M. A. , & Giannobile, W. V. (2004). Engineering of tooth‐supporting structures by delivery of PDGF gene therapy vectors. Molecular Therapy, 9(4), 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku, M. , Komatsu, Y. , Mochida, Y. , Yamauchi, M. , Mishina, Y. , & Ko, C.‐C. (2012). Identification and characterization of neural crest‐derived cells in adult periodontal ligament of mice. Archives of Oral Biology, 57(12), 1668–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J. , Fan, W. , Deng, Q. , He, H. , & Huang, F. (2019). Stem cells from the apical papilla: A promising source for stem cell‐based therapy. BioMed Research International, 2019, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao, R. T. , Nares, S. , & Reynolds, M. A. (2015). Periodontal regeneration ‐ intrabony defects: A systematic review from the AAP regeneration workshop. Journal of Periodontology, 86(2 Suppl), S77–S104. [DOI] [PubMed] [Google Scholar]
- Kassebaum, N. J. , Smith, A. G. C. , Bernabé, E. , Fleming, T. D. , Reynolds, A. E. , Vos, T. , … Yonemoto, N. (2017). Global, regional, and national prevalence, incidence, and disability‐adjusted life years for oral conditions for 195 countries, 1990–2015: A systematic analysis for the global burden of diseases, injuries, and risk factors. Journal of Dental Research, 96(4), 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Hong, H. S. , & Son, Y. (2016). Roles of endogenous growth factors and small peptides in in situ tissue regeneration. In In situ tissue regeneration (pp. 73–85). Cambridge, MA: Academic Press. [Google Scholar]
- Kitami, M. , Kaku, M. , Rocabado, J. M. R. , Ida, T. , Akiba, N. , & Uoshima, K. (2016). Prolonged survival of transplanted osteoblastic cells does not directly accelerate the healing of Calvarial bone defects. Journal of Cellular Physiology, 231(9), 1974–1982. [DOI] [PubMed] [Google Scholar]
- Kitamura, M. , Akamatsu, M. , Machigashira, M. , Hara, Y. , Sakagami, R. , Hirofuji, T. , … Murakami, S. (2011). FGF‐2 stimulates periodontal regeneration: Results of a multi‐center randomized clinical trial. Journal of Dental Research, 90(1), 35–40. [DOI] [PubMed] [Google Scholar]
- Klammert, U. , Mueller, T. D. , Hellmann, T. V. , Wuerzler, K. K. , Kotzsch, A. , Schliermann, A. , … Nickel, J. (2015). GDF‐5 can act as a context‐dependent BMP‐2 antagonist. BMC Biology, 13, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, F. P. , Becker, J. , Terheyden, H. , Capsius, B. , & Wagner, W. (2010). A prospective, randomized pilot study on the safety and efficacy of recombinant human growth and differentiation factor‐5 coated onto beta‐tricalcium phosphate for sinus lift augmentation. Clinical Oral Implants Research, 21(11), 1301–1308. [DOI] [PubMed] [Google Scholar]
- Kornman, K. S. , Giannobile, W. V. , & Duff, G. W. (2017). Quo vadis: What is the future of periodontics? How will we get there? Periodontology 2000, 75(1), 353–371. [DOI] [PubMed] [Google Scholar]
- Langer, R. , & Vacanti, J. (2016). Advances in tissue engineering. Journal of Pediatric Surgery, 51(1), 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer, R. , & Vacanti, J. P. (1993). Tissue engineering. Science, 260(5110), 920–926. [DOI] [PubMed] [Google Scholar]
- Lee, C. H. , Hajibandeh, J. , Suzuki, T. , Fan, A. , Shang, P. , & Mao, J. J. (2014). Three‐dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Engineering Part A, 20(7–8), 1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H. , Um, S. , Jang, J. H. , & Seo, B. M. (2012). Effects of VEGF and FGF‐2 on proliferation and differentiation of human periodontal ligament stem cells. Cell and Tissue Research, 348(3), 475–484. [DOI] [PubMed] [Google Scholar]
- Lekic, P. C. , Rajshankar, D. , Chen, H. , Tenenbaum, H. , & McCulloch, C. A. (2001). Transplantation of labeled periodontal ligament cells promotes regeneration of alveolar bone. The Anatomical Record, 262(2), 193–202. [DOI] [PubMed] [Google Scholar]
- Lin, H. , Chen, H. , Zhao, X. , Chen, Z. , Zhang, P. , Tian, Y. , … Shen, Y. (2021). Advances in mesenchymal stem cell conditioned medium‐mediated periodontal tissue regeneration. Journal of Translational Medicine, 19(1), 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhe, J. , Socransky, S. S. , Nyman, S. , Haffajee, A. , & Westfelt, E. (1982). "critical probing depths" in periodontal therapy. Journal of Clinical Periodontology, 9(4), 323–336. [DOI] [PubMed] [Google Scholar]
- Liu, S. H. , Yang, R. S. , Al‐Shaikh, R. , & Lane, J. M. (1995). Collagen in tendon, ligament, and bone healing. A current review. Clinical Orthopaedics and Related Research, 318, 265–278. [PubMed] [Google Scholar]
- Liu, X. , Pettway, G. J. , McCauley, L. K. , & Ma, P. X. (2007). Pulsatile release of parathyroid hormone from an implantable delivery system. Biomaterials, 28(28), 4124–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, P. X. (2008). Biomimetic materials for tissue engineering. Advanced Drug Delivery Reviews, 60(2), 184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegawa, N. , Kawamura, K. , Hirose, M. , Yajima, H. , Takakura, Y. , & Ohgushi, H. (2007). Enhancement of osteoblastic differentiation of mesenchymal stromal cells cultured by selective combination of bone morphogenetic protein‐2 (BMP‐2) and fibroblast growth factor‐2 (FGF‐2). Journal of Tissue Engineering and Regenerative Medicine, 1(4), 306–313. [DOI] [PubMed] [Google Scholar]
- Masset, A. , Staszyk, C. , & Gasse, H. (2006). The blood vessel system in the periodontal ligament of the equine cheek teeth – Part I: The spatial arrangement in layers. Annals of Anatomy, 188(6), 529–533. [DOI] [PubMed] [Google Scholar]
- McCulloch, C. A. , Barghava, U. , & Melcher, A. H. (1989). Cell death and the regulation of populations of cells in the periodontal ligament. Cell and Tissue Research, 255(1), 129–138. [DOI] [PubMed] [Google Scholar]
- McCulloch, C. A. , & Bordin, S. (1991). Role of fibroblast subpopulations in periodontal physiology and pathology. Journal of Periodontal Research, 26(3 Pt 1), 144–154. [DOI] [PubMed] [Google Scholar]
- McCulloch, C. A. , & Melcher, A. H. (1983). Cell migration in the periodontal ligament of mice. Journal of Periodontal Research, 18(4), 339–352. [DOI] [PubMed] [Google Scholar]
- McCulloch, C. A. , Lekic, P. , & McKee, M. D. (2000). Role of physical forces in regulating the form and function of the periodontal ligament. Periodontology 2000, 24(1), 56–72. [DOI] [PubMed] [Google Scholar]
- McKee, M. D. , Zalzal, S. , & Nanci, A. (1996). Extracellular matrix in tooth cementum and mantle dentin: Localization of osteopontin and other noncollagenous proteins, plasma proteins, and glycoconjugates by electron microscopy. The Anatomical Record, 245(2), 293–312. [DOI] [PubMed] [Google Scholar]
- Melcher, A. H. (1976). On the repair potential of periodontal tissues. Journal of Periodontology, 47(5), 256–260. [DOI] [PubMed] [Google Scholar]
- Menicanin, D. , Hynes, K. , Han, J. , Gronthos, S. , & Bartold, P. M. (2015). Cementum and periodontal ligament regeneration. Engineering Mineralized and Load Bearing Tissues, 881, 207–236. [DOI] [PubMed] [Google Scholar]
- Miron, R. J. , Bosshardt, D. D. , Hedbom, E. , Zhang, Y. , Haenni, B. , Buser, D. , & Sculean, A. (2012). Adsorption of enamel matrix proteins to a bovine‐derived bone grafting material and its regulation of cell adhesion, proliferation, and differentiation. Journal of Periodontology, 83(7), 936–947. [DOI] [PubMed] [Google Scholar]
- Miron, R. J. , Caluseru, O. M. , Guillemette, V. , Zhang, Y. , Gemperli, A. C. , Chandad, F. , & Sculean, A. (2013). Influence of enamel matrix derivative on cells at different maturation stages of differentiation. PLoS One, 8(8), e71008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron, R. J. , Sculean, A. , Cochran, D. L. , Froum, S. , Zucchelli, G. , Nemcovsky, C. , … Bosshardt, D. D. (2016). Twenty years of enamel matrix derivative: The past, the present and the future. Journal of Clinical Periodontology, 43(8), 668–683. [DOI] [PubMed] [Google Scholar]
- Mombelli, A. , Brochut, P. , Plagnat, D. , Casagni, F. , & Giannopoulou, C. (2005). Enamel matrix proteins and systemic antibiotics as adjuncts to non‐surgical periodontal treatment: Clinical effects. Journal of Clinical Periodontology, 32(3), 225–230. [DOI] [PubMed] [Google Scholar]
- Moore, Y. R. , Dickinson, D. P. , & Wikesjö, U. M. E. (2010). Growth/differentiation factor‐5: A candidate therapeutic agent for periodontal regeneration? A review of pre‐clinical data. Journal of Clinical Periodontology, 37(3), 288–298. [DOI] [PubMed] [Google Scholar]
- Morotome, Y. , Goseki‐Sone, M. , Ishikawa, I. , & Oida, S. (1998). Gene expression of growth and differentiation factors‐5, −6, and −7 in developing bovine tooth at the root forming stage. Biochemical and Biophysical Research Communications, 244(1), 85–90. [DOI] [PubMed] [Google Scholar]
- Murakami, S. (2011). Periodontal tissue regeneration by signaling molecule(s): What role does basic fibroblast growth factor (FGF‐2) have in periodontal therapy? Periodontology 2000, 56(1), 188–208. [DOI] [PubMed] [Google Scholar]
- Nakamura, Y. , Tensho, K. , Nakaya, H. , Nawata, M. , Okabe, T. , & Wakitani, S. (2005). Low dose fibroblast growth factor‐2 (FGF‐2) enhances bone morphogenetic protein‐2 (BMP‐2)‐induced ectopic bone formation in mice. Bone, 36(3), 399–407. [DOI] [PubMed] [Google Scholar]
- Naqvi, S. M. , & McNamara, L. M. (2020). Stem cell mechanobiology and the role of biomaterials in governing mechanotransduction and matrix production for tissue regeneration. Frontiers in Bioengineering and Biotechnology, 8, 597661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan, R. , Huang, C. C. , & Ravindran, S. (2016). Hijacking the cellular mail: Exosome mediated differentiation of mesenchymal stem cells. Stem Cells International, 2016, 3808674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins, M. , Giannobile, W. V. , McGuire, M. K. , Kao, R. T. , Mellonig, J. T. , Hinrichs, J. E. , … Lynch, S. E. (2005). Platelet‐derived growth factor stimulates bone fill and rate of attachment level gain: Results of a large multicenter randomized controlled trial. Journal of Periodontology, 76(12), 2205–2215. [DOI] [PubMed] [Google Scholar]
- Gu, D.‐I. , Nguyen, T. , Gonzalez, A. M. , Printz, M. A. , Pierce, G. F. , Sosnowski, B. A. , … Chandler, L. A. (2004). Adenovirus encoding human platelet‐derived growth factor‐B delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Molecular Therapy, 9(5), 699–711. [DOI] [PubMed] [Google Scholar]
- Nishitoh, H. , Ichijo, H. , Kimura, M. , Matsumoto, T. , Makishima, F. , Yamaguchi, A. , … Miyazono, K. (1996). Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor‐5. The Journal of Biological Chemistry, 271(35), 21345–21352. [DOI] [PubMed] [Google Scholar]
- Nyman, S. , Lindhe, J. , Karring, T. , & Rylander, H. (1982). New attachment following surgical treatment of human periodontal disease. Journal of Clinical Periodontology, 9(4), 290–296. [DOI] [PubMed] [Google Scholar]
- Pagella, P. , de Vargas Roditi, L. , Stadlinger, B. , Moor, A. E. , & Mitsiadis, T. A. (2021). A single‐cell atlas of human teeth. iScience, 24(5), 102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, R. M. , & Lumsden, A. G. (1987). Development of periodontal ligament and alveolar bone in homografted recombinations of enamel organs and papillary, pulpal and follicular mesenchyme in the mouse. Archives of Oral Biology, 32(4), 281–289. [DOI] [PubMed] [Google Scholar]
- Park, C. H. , Rios, H. F. , Jin, Q. , Sugai, J. V. , Padial‐Molina, M. , Taut, A. D. , … Giannobile, W. V. (2012). Tissue engineering bone‐ligament complexes using fiber‐guiding scaffolds. Biomaterials, 33(1), 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz, A. , Albuquerque‐Souza, E. , Gasparoni, L. M. , França, B. N. D. , Pelissari, C. , Trierveiler, M. , & Holzhausen, M. (2021). Therapeutic potential of periodontal ligament stem cells. World Journal of Stem Cells, 13(6), 605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorth, M. , & Narvekar, A. (2015). Non viral vectors in gene therapy‐an overview. Journal of Clinical and Diagnostic Research: JCDR, 9(1), GE01–GE06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambhia, K. J. , & Ma, P. X. (2015). Controlled drug release for tissue engineering. Journal of Controlled Release, 219, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, X. , Zhao, M. , Lash, B. , Martino, M. M. , & Julier, Z. (2020). Growth factor engineering strategies for regenerative medicine applications. Frontiers in Bioengineering and Biotechnology, 7, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, M. A. , Kao, R. T. , Camargo, P. M. , Caton, J. G. , Clem, D. S. , Fiorellini, J. P. , … Nevins, M. L. (2015). Periodontal regeneration ‐ intrabony defects: A consensus report from the AAP regeneration workshop. Journal of Periodontology, 86(2 Suppl), S105–S107. [DOI] [PubMed] [Google Scholar]
- Rubins, R. , Tolmie, P. , Corsig, K. , Kerr, E. , & Kim, D. (2014). Subepithelial connective tissue graft with purified rhPDGF‐BB for the treatment of mandibular recession defects: A consecutive case series. International Journal of Periodontics & Restorative Dentistry, 34(3), 315–321. [DOI] [PubMed] [Google Scholar]
- Rutkowski, J. L. (2018). Implant success and failure is dependent upon the bone response. Show a little respect for those bone cells! Journal of Oral Implantology, 44(2), 85–86. [DOI] [PubMed] [Google Scholar]
- Seo, B. M. , Miura, M. , Gronthos, S. , Bartold, P. M. , Batouli, S. , Brahim, J. , … Shi, S. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet, 364(9429), 149–155. [DOI] [PubMed] [Google Scholar]
- Seo, B. M. , Miura, M. , Sonoyama, W. , Coppe, C. , Stanyon, R. , & Shi, S. (2005). Recovery of stem cells from cryopreserved periodontal ligament. Journal of Dental Research, 84(10), 907–912. [DOI] [PubMed] [Google Scholar]
- Shinmura, Y. , Tsuchiya, S. , Hata, K. , & Honda, M. J. (2008). Quiescent epithelial cell rests of Malassez can differentiate into ameloblast‐like cells. Journal of Cellular Physiology, 217(3), 728–738. [DOI] [PubMed] [Google Scholar]
- Somerman, M. (2010). Growth factors and periodontal engineering. Journal of Dental Research, 90(1), 7–8. [DOI] [PubMed] [Google Scholar]
- Sowmya, S. , Mony, U. , Jayachandran, P. , Reshma, S. , Kumar, R. A. , Arzate, H. , … Jayakumar, R. (2017). Tri‐layered nanocomposite hydrogel scaffold for the concurrent regeneration of cementum, periodontal ligament, and alveolar bone. Advanced Healthcare Materials, 6(7), 1601251. [DOI] [PubMed] [Google Scholar]
- Stambaugh, R. V. , Dragoo, M. , Smith, D. M. , & Carasali, L. (1981). The limits of subgingival scaling. The International Journal of Periodontics & Restorative Dentistry, 1(5), 30–41. [PubMed] [Google Scholar]
- Stavropoulos, A. , Becker, J. , Capsius, B. , Acil, Y. , Wagner, W. , & Terheyden, H. (2011). Histological evaluation of maxillary sinus floor augmentation with recombinant human growth and differentiation factor‐5‐coated beta‐tricalcium phosphate: Results of a multicenter randomized clinical trial. Journal of Clinical Periodontology, 38(10), 966–974. [DOI] [PubMed] [Google Scholar]
- Stavropoulos, A. , Windisch, P. , Gera, I. , Capsius, B. , Sculean, A. , & Wikesjo, U. M. (2011). A phase IIa randomized controlled clinical and histological pilot study evaluating rhGDF‐5/beta‐TCP for periodontal regeneration. Journal of Clinical Periodontology, 38(11), 1044–1054. [DOI] [PubMed] [Google Scholar]
- Storm, E. E. , & Kingsley, D. M. (1999). GDF5 coordinates bone and joint formation during digit development. Developmental Biology, 209(1), 11–27. [DOI] [PubMed] [Google Scholar]
- Suarez‐Lopez Del Amo, F. , Monje, A. , Padial‐Molina, M. , Tang, Z. , & Wang, H. L. (2015). Biologic agents for periodontal regeneration and implant site development. BioMed Research International, 2015, 957518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, W. B. , Gong, T. , Zhang, Z. , Eberle, M. , Niemann, D. , Dong, R. , … Ma, P. X. (2020). Controlled release of odontogenic exosomes from a biodegradable vehicle mediates dentinogenesis as a novel biomimetic pulp capping therapy. Journal of Controlled Release, 324, 679–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, W. B. , & Ma, P. X. (2020). Nanofibrous and porous biomaterials. In Zhang G. (Ed.), Biomaterials science, An introduction to materials in medicine. Amsterdam, Netherlands: Elsevier. [Google Scholar]
- Swanson, W. B. , & Mishina, Y. (2022). New paradigms in regenerative engineering: Emerging role of extracellular vesicles paired with instructive biomaterials. Biocell, 46(6), 1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, W. B. , Omi, M. , Woodbury, S. M. , Douglas, L. M. , Eberle, M. , Ma, P. X. , … Mishina, Y. (2022). Scaffold pore curvature influences ΜSC fate through differential cellular organization and YAP/TAZ activity. International Journal of Molecular Sciences, 23(9), 4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, W. B. , Omi, M. , Zhang, Z. , Nam, H. K. , Jung, Y. , Wang, G. , … Mishina, Y. (2021). Macropore Design of Tissue Engineering Scaffolds Regulates Mesenchymal Stem Cell Differentiation Fate. Biomaterials, 272, 120769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, W. B. , Zhang, Z. , Xiu, K. , Gong, T. , Eberle, M. , Wang, Z. , & Ma, P. X. (2020). Scaffolds with controlled release of pro‐mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomaterialia, 118, 215–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, Z. , Zhao, Q. , Gong, P. , Wu, Y. , Wei, N. , Yuan, Q. , … Tang, H. (2009). Research on promoting periodontal regeneration with human basic fibroblast growth factor‐modified bone marrow mesenchymal stromal cell gene therapy. Cytotherapy, 11(3), 317–325. [DOI] [PubMed] [Google Scholar]
- Ten Cate, A. R. (1998). Oral histology: Development, structure, and function (5th ed.). St. Louis, MO: Mosby. [Google Scholar]
- Tete, S. , Mastrangelo, F. , Bianchi, A. , Zizzari, V. , & Scarano, A. (2009). Collagen fiber orientation around machined titanium and zirconia dental implant necks: An animal study. The International Journal of Oral & Maxillofacial Implants, 24(1), 52–58. [PubMed] [Google Scholar]
- Tonetti, M. S. , Greenwell, H. , & Kornman, K. S. (2018). Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of Periodontology, 89, S159–S172. [DOI] [PubMed] [Google Scholar]
- Traini, T. , Degidi, M. , Strocchi, R. , Caputi, S. , & Piattelli, A. (2005). Collagen fiber orientation near dental implants in human bone: Do their organization reflect differences in loading? Journal of Biomedical Materials Research. Part B, Applied Biomaterials, 74(1), 538–546. [DOI] [PubMed] [Google Scholar]
- Tsunematsu, T. , Fujiwara, N. , Yoshida, M. , Takayama, Y. , Kujiraoka, S. , Qi, G. , … Kudo, Y. (2016). Human odontogenic epithelial cells derived from epithelial rests of Malassez possess stem cell properties. Laboratory Investigation, 96(10), 1063–1075. [DOI] [PubMed] [Google Scholar]
- Van Steenberghe, D. (2000). From osseointegration to osseoperception. Journal of Dental Research, 79(11), 1833–1837. [DOI] [PubMed] [Google Scholar]
- Vaquette, C. , Fan, W. , Xiao, Y. , Hamlet, S. , Hutmacher, D. W. , & Ivanovski, S. (2012). A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials, 33(22), 5560–5573. [DOI] [PubMed] [Google Scholar]
- Wada, N. , Menicanin, D. , Shi, S. , Bartold, P. M. , & Gronthos, S. (2009). Immunomodulatory properties of human periodontal ligament stem cells. Journal of Cellular Physiology, 219(3), 667–676. [DOI] [PubMed] [Google Scholar]
- Wei, G. , Jin, Q. , Giannobile, W. V. , & Ma, P. X. (2006). Nano‐fibrous scaffold for controlled delivery of recombinant human PDGF‐BB. Journal of Controlled Release, 112(1), 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikesjo, U. M. , Sigurdsson, T. J. , Lee, M. B. , Tatakis, D. N. , & Selvig, K. A. (1995). Dynamics of wound healing in periodontal regenerative therapy. Journal of the California Dental Association, 23(12), 30–35. [PubMed] [Google Scholar]
- Wikesjo, U. M. , Sorensen, R. G. , Kinoshita, A. , Jian Li, X. , & Wozney, J. M. (2004). Periodontal repair in dogs: Effect of recombinant human bone morphogenetic protein‐12 (rhBMP‐12) on regeneration of alveolar bone and periodontal attachment. Journal of Clinical Periodontology, 31(8), 662–670. [DOI] [PubMed] [Google Scholar]
- Wolff, L. F. (2000). Guided tissue regeneration in periodontal therapy. Northwest Dent, 79(6), 23–28. [PubMed] [Google Scholar]
- Woo, H. N. , Cho, Y. J. , Tarafder, S. , & Lee, C. H. (2021). The recent advances in scaffolds for integrated periodontal regeneration. Bioactive Materials, 6(10), 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, X. Z. , van den Beucken, J. J. , Cai, X. , Yu, N. , Jansen, J. A. , & Yang, F. (2015). Periodontal tissue regeneration using enzymatically solidified chitosan hydrogels with or without cell loading. Tissue Engineering. Part A, 21(5–6), 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, X.‐Z. , Yang, F. , Jansen, J. A. , de Vries, R. B. M. , & van den Beucken, J. J. J. P. (2015). Cell‐based approaches in periodontal regeneration: A systematic review and meta‐analysis of periodontal defect models in animal experimental work. Tissue Engineering Part B: Reviews, 21(5), 411–426. [DOI] [PubMed] [Google Scholar]
- Yang, H. , Gao, L. N. , An, Y. , Hu, C. H. , Jin, F. , Zhou, J. , … Chen, F. M. (2013). Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials, 34(29), 7033–7047. [DOI] [PubMed] [Google Scholar]
- Yao, Y. , Raymond, J. E. , Kauffmann, F. , Maekawa, S. , Sugai, J. V. , Lahann, J. , & Giannobile, W. V. (2022). Multicompartmental Scaffolds for coordinated periodontal tissue engineering. Journal of Dental Research. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuda, A. , Maeda, H. , Fujii, S. , Monnouchi, S. , Yamamoto, N. , Wada, N. , … Akamine, A. (2015). Effect of CTGF/CCN2 on Osteo/Cementoblastic and fibroblastic differentiation of a human periodontal ligament stem/progenitor cell line. Journal of Cellular Physiology, 230(1), 150–159. [DOI] [PubMed] [Google Scholar]
- Yue, C. , Cao, J. , Wong, A. , Kim, J. H. , Alam, S. , Luong, G. , … Lin, Z. (2022). Human bone marrow stromal cell exosomes ameliorate periodontitis. Journal of Dental Research, 101, 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R. , & Ma, P. X. (2000). Synthetic nano‐fibrillar extracellular matrices with predesigned macroporous architectures. Journal of Biomedical Materials Research, 52(2), 430–438. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , & Yelick, P. C. (2021). Tooth repair and regeneration: Potential of dental stem cells. Trends in Molecular Medicine, 27(5), 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Liao, K. , Li, C. , Lai, A. , Foo, J.‐J. , & Chan, V. (2017). Progress in integrative biomaterial systems to approach three‐dimensional cell mechanotransduction. Bioengineering, 4(4), 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Miron, R. J. , Li, S. , Shi, B. , Sculean, A. , & Cheng, X. (2015). Novel M eso P orous B ioGlass/silk scaffold containing ad PDGF‐B and ad BMP 7 for the repair of periodontal defects in beagle dogs. Journal of Clinical Periodontology, 42(3), 262–271. [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Volponi, A. A. , Caetano, A. , & Sharpe, P. T. (2020). Mesenchymal stem cells in teeth. In Encyclopedia of bone biology (pp. 109–118). Amsterdam, Netherlands: Elsevier. [Google Scholar]
- Zheng, W. , Wang, S. , Ma, D. , Tang, L. , Duan, Y. , & Jin, Y. (2009). Loss of proliferation and differentiation capacity of aged human periodontal ligament stem cells and rejuvenation by exposure to the young extrinsic environment. Tissue Engineering. Part A, 15(9), 2363–2371. [DOI] [PubMed] [Google Scholar]
- Zhu, W. , & Liang, M. (2015). Periodontal ligament stem cells: Current status, concerns, and future prospects. Stem Cells International, 2015, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data related to this manuscript are provided in relevant citations and included in the manuscript.


