Table 3.
Summary of MCL1 inhibitors under clinical trials.
| Compound, chemical structure, and institution | Potency (TR‐FRET) | Clinical trials a | Study | Administration | Indication | References |
|---|---|---|---|---|---|---|
|
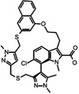
AZD5991 AstraZeneca |
MCL1 K i = 0.2 nm BCL2 K i = 6.8 μm BCL‐xL K i = 18 μm BCLW K i = 25 μm BFL1 K i = 12 μm |
NCT03218683 | Phase I, monotherapy, and combination | IV | Refractory Hematological malignancies | [213] |
|
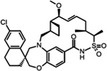
AMG‐176 Amgen |
MCL1 K i = 0.06 nm BCL2 K i = 0.95 μm BCL‐xL K i = 0.7 μm |
NCT02675452 | Phase I, monotherapy | IV | Relapsed or refractory MM/AML | [159] |
| NCT03797261 | Phase I, monotherapy with venetoclax | IV | AML/Lymphoma | |||
|
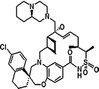
AMG‐397 Amgen |
Not disclosed | NCT03465540 | Phase I, monotherapy | Oral | MM/AML/NHL | [214] |
|
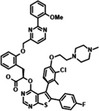
S64315/MIK665 Servier/Novartis |
MCL1 K i = 1.2 nm | NCT02979366 | Phase I, monotherapy | IV | AML/MDS | [156] |
| NCT02992483 | Phase I, monotherapy | IV | MM/Lymphoma | |||
| NCT03672695 | Phase I, combination with venetoclax | IV | AML/MDS | |||
| NCT04629443 | Phase II, combination with azacitidine | IV | AML | |||
| NCT04702425 | Phase I, combination with VOB560 | IV | NHL/AML/MM | |||
|
Structure not disclosed ABBV‐467 AbbVie |
Not disclosed | NCT04178902 | Phase I, monotherapy | IV | MM | Not disclosed |
|
Structure not disclosed PRT1419 Prelude Therapeutics |
Not disclosed | NCT04543305 | Phase I, monotherapy | Oral | Refractory Hematological malignancies | Not disclosed |
| NCT04837677 | Phase I, monotherapy | IV | Relapsed or refractory solid tumors |
From www.ClinicalTrials.gov.