Fig. 3.
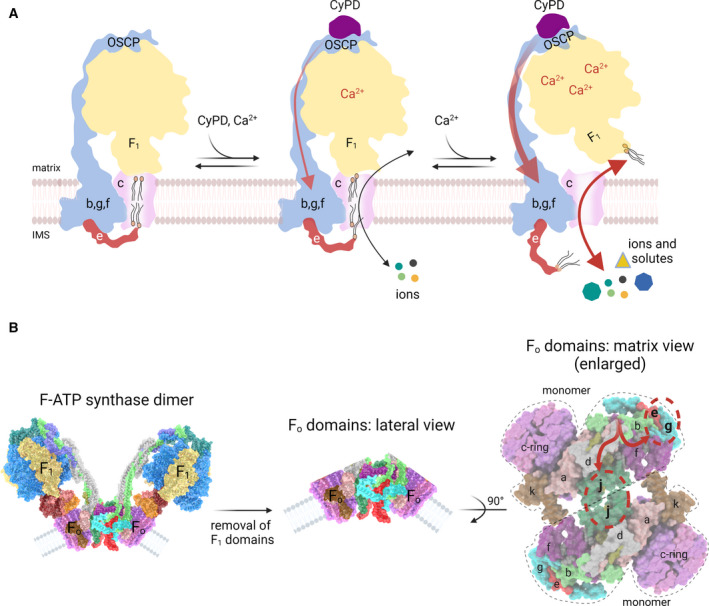
Hypothetical models for PTP formation by F‐ATP synthase. (A) The ‘death finger’ model proposes the transition of the c‐ring into the PTP channel on a conformational change of the peripheral stalk that eventually perturbs subunit e, the final transducer of pore opening. The C‐terminus of subunit e makes contacts with lipids of the plug within the c‐ring from the IMS (left). In the presence of physiological, low Ca2+ concentrations with CyPD bound to OSCP subunit (middle), the peripheral stalk transmits mechanical force generated by Ca2+ binding to subunit e (red arrow), which may exert a pulling action dragging some lipids out of the plug. This condition, together with a secondary relaxation of the central stalk/c‐ring connections would accommodate a low‐conductance mode of channel opening mediating the passage of ions but not of larger solutes, representing the ‘flickering’ mode of the PTP (middle). As the matrix Ca2+ levels rise, the mechanical force exerted on subunit e becomes stronger allowing removal of the lipid plug from the c‐ring and displacement of F 1 with formation of the high‐conductance PTP, which remains fully reversible if Ca2+ is removed [145]. (B) Entire F‐ATP synthase dimer (left), after removal of the F 1 domains (centre) and following a 90° rotation to show the two F O domains as viewed from the matrix side (right). The conformational change transmitted through the peripheral stalk (red arrows in the right panel) may affect both subunits g/e and the monomer–monomer interface, with possible channel formation at the point of contact of subunits j, which undergo a pivoting motion at their interface during catalysis [124].
