FIGURE 3.
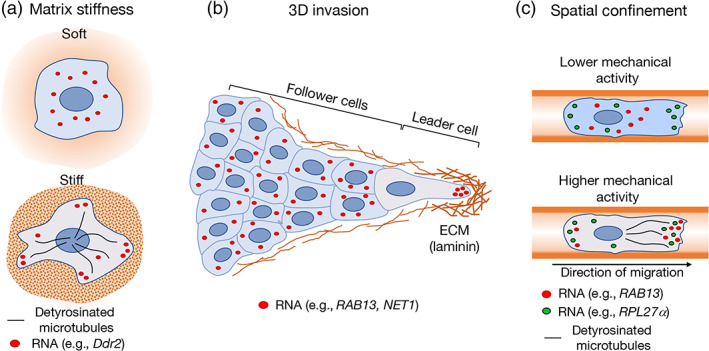
Mechanical regulation of mRNA localization. (a) The stiffness of the extracellular matrix modulates the distribution of cytosolic mRNAs. Stiff environments promote protrusion localization of APC‐dependent mRNAs (such as Ddr2) via a RhoA‐dependent mechanism that involves detyrosination of the microtubule network. (b) Localization patterns differ among cells in collectively invading 3D groups. During collective invasion of cancer cells, APC‐dependent mRNAs (such as NET1 and RAB13) localize prominently at the front of leader cells. This localization requires integrin‐mediated adhesion to the extracellular matrix (ECM) and coincides with a local accumulation of extracellular laminin. Notably, such an mRNA localization pattern is not observed in follower cells. (c) Spatial confinement modulates mRNA localization patterns. Ribosomal protein mRNAs (such as RPL27α) become more enriched at peripheral protrusions of cells migrating in confining microchannels. The localization of other mRNAs in confinement depends on the mechanical state of the cell. APC‐dependent mRNAs (such as RAB13) become preferentially enriched at leading protrusions of cell types that exhibit higher mechanical activity and stable, detyrosinated microtubules. This localization pattern can contribute to the efficiency of cell migration through confined spaces
