Abstract
An adenoviral (AdV)-based vector system is a promising platform for vaccine development and gene therapy applications. Administration of an AdV vector elicits robust innate immunity, leading to the development of humoral and cellular immune responses against the vector and the transgene antigen, if applicable. The use of high doses (1011–1013 virus particles) of an AdV vector, especially for gene therapy applications, could lead to vector toxicity due to excessive levels of innate immune responses, vector interactions with blood factors, or high levels of vector transduction in the liver and spleen. Additionally, the high prevalence of AdV infections in humans or the first inoculation with the AdV vector result in the development of vector-specific immune responses, popularly known as preexisting vector immunity. It significantly reduces the vector efficiency following the use of an AdV vector that is prone to preexisting vector immunity. Several approaches have been developed to overcome this problem. The utilization of rare human AdV types or nonhuman AdVs is the primary strategy to evade preexisting vector immunity. The use of heterologous viral vectors, capsid modification, and vector encapsulation are alternative methods to evade vector immunity. The vectors can be optimized for clinical applications with comprehensive knowledge of AdV vector immunity, toxicity, and circumvention strategies.
Keywords: adenoviral vector immunity, preexisting immunity, adenoviral immunity, circumvention of preexisting vector immunity, adenovirus capsid, adenovirus tropism, adenoviral innate immunity, hepatic toxicity, blood factor, adenoviral vector vaccine, adenoviral gene therapy
1. Introduction
Adenovirus (AdV) was first isolated in 1953 from human adenoids while searching for the etiologic agent of acute respiratory infections [1,2], and it was later characterized as human Ad (HAdV) type 5 (HAd5). Currently, more than 100 genotypes of HAdVs are known [3,4]. Overall, AdV-based vectors can infect several types of rapidly dividing or quiescent cells [5]. They can easily be propagated to high titers and purified in large quantities and support the high-level expression of a foreign gene. Activation of innate immunity following AdV vector inoculation leads to antigen-specific humoral and cell-mediated immune responses. Therefore, AdV-based vectors provide an attractive platform for recombinant vaccines and gene therapy.
Vaccines play a vital role in public health by significantly protecting humans and animals from infectious diseases. Most of the currently licensed human vaccines are conventional vaccines in the form of live-attenuated, inactivated, or subunit vaccines. During the COVID-19 pandemic, second-generation vaccine platforms such as AdV vector-based and mRNA-based vaccines were developed [6,7]. An AdV vector for gene therapy can play different roles depending on the need, including the somatic cell expression of a defective gene for correcting metabolic or neurologic disorders [8,9,10,11,12], the use of a tumor suppressor or apoptosis gene [12,13,14], immunomodulation of the host’s immune system [15], induction of immune responses against cancer antigens [16], and selective cell lysis with a conditional replication-competent vector [17]. Invariably, the vector dose for a gene therapy application is many-fold higher than the vector for a vaccine. Therefore, systemic delivery of high doses of AdV vectors for gene therapy can result in vector toxicity in patients [18].
The high prevalence of AdV infections in humans results in preexisting AdV immunity, which hinders the efficacy of many HAdV vectors [19]. Moreover, inoculation with an AdV vector will also elicit anti-vector immunity, which may significantly inhibit its effectiveness following the repeat administration of the same vector [20].
2. AdV Biology
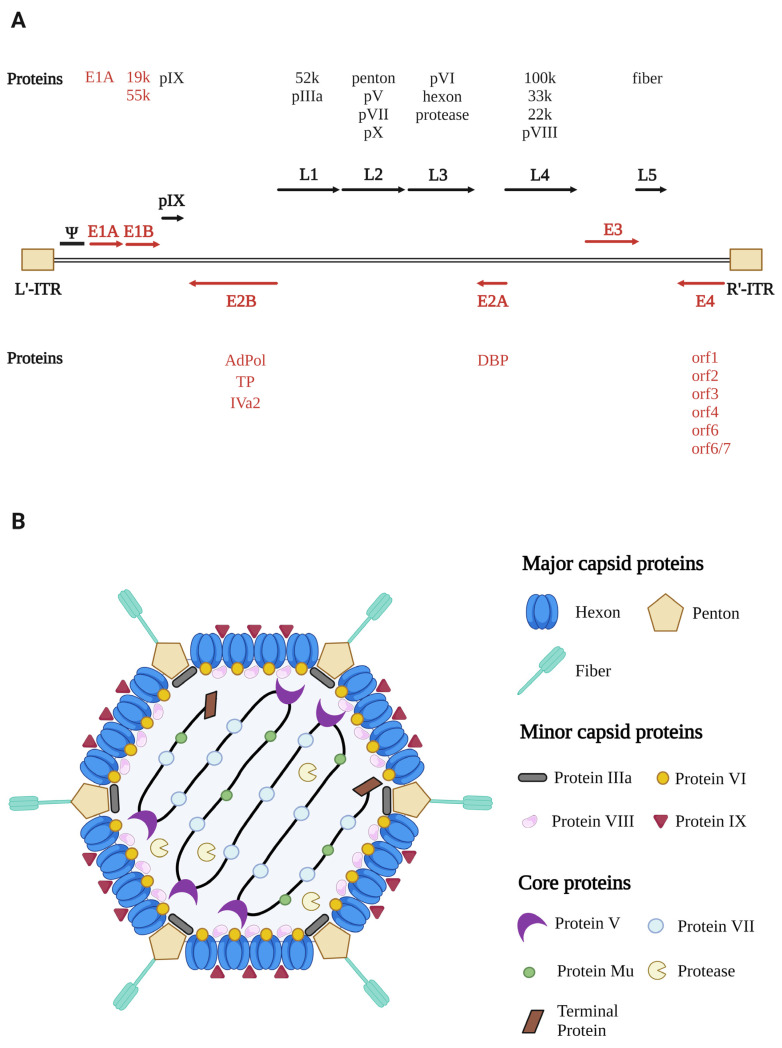
AdVs are approximately 90–100 nm in diameter and are nonenveloped icosahedral viruses belonging to the Adenoviridae family [21], consisting of double-stranded linearized DNA genomes in a range of 25–48 kilobase pairs (Figure 1A). The genome has inverted terminal repeat (ITR) sequences in the range of 26–721 bp [3], depending on the AdV type and the species of origin, at the left and right ends of the genomes, which function as the origins of DNA replication [22]. Next to the left ITR, the genome packaging signal is essential for packaging the AdV genome [23]. The early regions (E1, E2, E3, and E4) encode proteins critical for activating the transcription of other viral genes, preparing the host cell for virus replication, or modulating host immune responses [24,25,26]. The late regions (L1-L5) encode most structural proteins to produce progeny virions [22,24,25].
Figure 1.
Genome organization and structure of human adenovirus type 5. (A) Schematic representation of the adenoviral genome, transcription units, and major proteins. (B) Structural representation of adenovirus and its components. E, early region (shown as red arrows); L, late region (shown as black arrows); L’-ITR, left inverted terminal repeat; R’-ITR, right inverted terminal repeat; Ψ, packaging signal; AdPol, adenovirus DNA polymerase; TP, terminal protein; DBP, DNA-binding protein.
The hexon (protein II), penton base (protein III), and fiber (protein IV) are the major capsid proteins that create the capsid shell [27]. The 240 hexons form the facet of the icosahedron (12 per facet) and 12 penton bases lie at each vertex of the icosahedron with extensions of 12 fibers [28]. The fiber protein comprises knob and stem domains, and the fiber length varies among AdV types [29]. All major capsid proteins are the targets for neutralizing antibodies; however, the hexon serves as the dominant immunogen for type-specific neutralizing antibodies [30,31]. Four minor proteins (protein IIIa, VI, VIII, IX), known as cementing proteins, help to assemble and support the AdV virion structure at both the inner and outer surfaces [32,33]. The VI and VI precursor proteins (pVI) play critical roles in the endosome escape of AdV after endocytosis and activate the cleavage of several precursor proteins [34]. Moreover, six core proteins (V, VII, IVa2, terminal protein, Mu, and protease) are responsible for DNA replication and transcription, DNA condensation, and viral assembly [33,35]. A schematic virion structure of HAdV5 consisting of at least 13 proteins is presented (Figure 1B). According to the phylogenetic classification, genome organization, and biological features, AdVs are classified into six genera: Mastadenovirus, Aviadenovirus, Atadenovirus, Siadenovirus, Ichtadenovirus, and Testadenovirus [3]. Furthermore, based on their hemagglutination properties, sequence correlation, and oncogenic characteristics, HAdVs are categorized into seven species (A-G) [3,36,37].
3. AdV Receptors and Cell Entry
AdVs utilize the distal knob domain to bind to their respective primary cell receptors with high affinity and initiate endocytosis (Figure 2) [38,39]. Many HAdVs recognize the Coxsackie and AdV Receptor (CAR) on the cell surface for attachment and internalization [40]. CAR is a transmembrane glycoprotein consisting of two immunoglobulin-like extracellular domains, a transmembrane domain, and a long cytoplasmic domain. The immunoglobulin-like domain (D1) seems necessary for viral binding [41]. CAR is a component at the tight junction of epithelial cells mediating cell–cell adhesion and is broadly expressed in different tissues [40,42]. CAR expression on non-permissive cells increases the repertoire of AdV-mediated gene transfer [43,44]. Meanwhile, type B HAdVs utilize CD46, CD80/86, or receptor X as the cell receptor for attachment [45,46,47,48,49,50]. Moreover, some AdVs use receptors other than CAR for cell entry, such as desmoglein-2 for HAdV3, 7, 11, 14 [51], glycans GD1a for HAdV37 [52], and sialic acid for HAdV37 and 52 [53,54]. AdVs of different species, such as bovine, porcine, or ovine, use CAR-independent mechanisms for cell attachment and internalization [55,56,57,58].
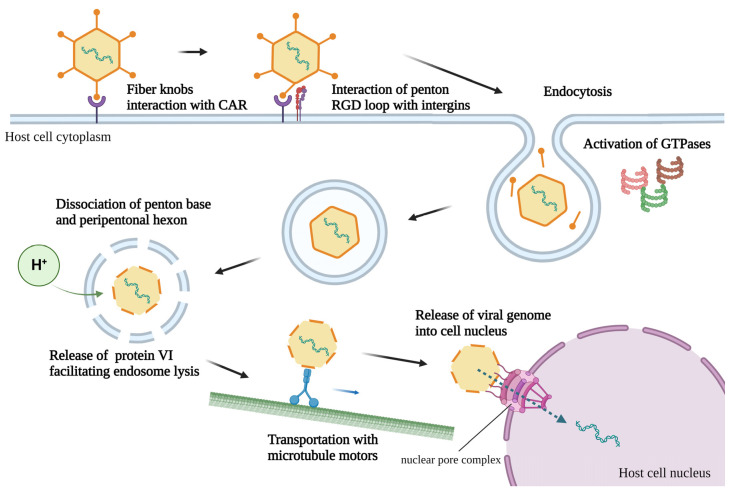
Figure 2.
Transportation of human adenovirus type 5 or its genome within a host cell. The adenoviral knob domain binds to the CAR on the host cell surface. Following receptor binding, the fibers start to disassociate, exposing the RGD loop of the penton to interact with αv integrins, thereby initiating endocytosis. The endosome’s acidic environment results in the dissociation of the penton base and peripentonal hexon, followed by protein VI-mediated lysis of the endosome. The viral particle is transported to the nuclear membrane by microtubule motors. Finally, the adenoviral genome is transported to the nucleus through the nuclear pore complex. CAR, Coxsackievirus and adenovirus receptor; RGD, arginine–glycine–aspartic acid; GTPase; guanosine triphosphate hydrolase.
Interaction between CAR and AdV fiber knobs displaces the fibers, thereby exposing the penton base [42,59]. It allows the attachment of the RGD domain of a penton with αv integrins, a heterodimeric transmembrane protein involved in cell adhesion, and stimulates virus internalization by facilitating clathrin-coated endocytosis [60,61]. Subsequently, the activation of several GTPases, such as dynamin, PI3K, Rac1, Cdc42, and Rab5, results in virus internalization into the clathrin-coated endosome [39,62,63]. The acidic environment in the endosome results in the dissociation of the penton base and peripentonal hexon from the virion, followed by protein VI-mediated endosomal lysis [61,64]. The microtubule motors transport the uncoated viral particle to the nuclear membrane. Subsequently, the viral genome is injected into the nucleus via the nuclear pore complex [65,66].
4. AdV Vector Construction
AdVs’ genome manipulation for vector generation for vaccine development and gene therapy applications has evolved over the years [14,67]. Initially, the replication-competent AdV vector was generated by deleting the E3 region, which is not essential for virus replication [68]. The deletion of E1 and E3 was introduced into the AdV vector to increase the size of the foreign gene cassette, and this type of replication-defective vector is considered the first-generation AdV vector [69]. The E1- and E3-deleted vectors can easily be propagated in cell lines that constitutively express E1 proteins, such as HEK 293 [70] and PER.C6 [71]. Several techniques have been developed to construct AdV vectors. The investigators have used homologous recombination in bacteria [72], the Cre-lox recombination system [73], and homologous recombination in the appropriate cell lines [74] to generate AdV vectors. Due to the leaky expression of some of the AdV proteins, the development of AdV-specific immune responses at high levels will eliminate the transduced cells, thereby shortening the duration of transgene expression [75]. Therefore, the second generation of AdV vectors was developed by extending deletions in the E2 and/or E4 regions, in addition to E1 and E3 deletions. These vectors have a foreign gene insertion capacity of up to 14 kb, with improved vector survival in the host [76,77].
Nevertheless, the vector yield per cell is less than in the first-generation vectors because of the reduced replication in the producer cell lines [67]. The third-generation AdV vectors, known as helper-dependent or gutless AdV vectors, were generated by the deletion of the viral genome except for the ITRs and packaging signal. A particular AdV helper virus is used for generating helper-dependent AdV vectors having a larger insert capacity with low toxicity [78,79,80]. Moreover, the conditional replication-competent AdV vectors or oncolytic AdV vectors can be generated by controlling the E1 expression with an inducible promoter [17]. The first-generation vectors are preferred for vaccine development, while the second, third, and conditional replication-competent AdV vectors are better suited for gene therapy. The implications of oncolytic AdV vectors have been reviewed in detail elsewhere [81,82].
Several unique characteristics of AdV vectors have contributed to their potential as vectors for gene delivery. The AdV genome can easily be manipulated, allowing the insertion of a sizeable foreign gene cassette [67], which remains stable even after multiple passages, and the vector system has an excellent safety record [83,84]. AdV vectors contain various pathogen-associated molecular patterns (PAMPs) interacting with pathogen recognition receptors (PRR), thereby stimulating robust innate immune responses leading to enhanced adaptive immunity [36,85,86,87]. Moreover, AdV vectors can quickly be grown and purified in large quantities and have broad host cell tropism [5,88]. Furthermore, AdV vector vaccines can be administrated through the mucosal route, such as the oral and the nasal routes [89]. AdV vector vaccines administered through the mucosal surfaces can induce cellular and mucosal immunity [89]. Mucosal immunity can prevent the initial entrance of pathogens, which is a great advantage against respiratory pathogens [90,91].
5. AdV-Mediated Activation of Innate Immunity
The host immune system can recognize PAMPs in microbial structures or products through PRRs on the cell surface, resulting in an innate immunity cascade [87,92]. One of the best-characterized types of PRRs is Toll-like receptors (TLRs), which are expressed on antigen-presenting cells (dendritic cells and macrophages) as well as non-immune cells such as fibroblasts and epithelial cells [93]. The presence of various PAMPs in AdVs results in the activation of multiple innate immune pathways [36,86]. The innate immunity is initiated by the sensing of AdV through interactions between viral proteins and cellular receptors. The binding of viral fiber to CAR (with many AdVs) and/or interactions between the penton base and integrins induce the downstream signaling of nuclear factor kappa B (NF-κB), resulting in the increased expression of proinflammatory cytokines and chemokines [94,95,96]. In addition, the binding of the blood coagulation factor X (FX) to the AdV hexon is associated with the activation of TLR4 adaptor molecules such as differentiation primary response 88 (MyD88), TNF receptor-associated factor 6 (TRAF6), and TIR domain-containing adaptor-inducing interferon-β (TRIF), following the stimulation of TLR4 [97]. Upregulation of NF-κB results in the enhanced expression of several proinflammatory cytokines and chemokines, such as IL1α, IL1β, IFNα, TNFα, IL2, IL6, IL8, CCL2, CCL3, CCL4, CXCL1, CXCL2, CXCL10, GM-CSF, and others [97,98,99]. The levels of cytokines’ and chemokines’ expression are dependent on the AdV vector dose and the host. In a mouse model, the elevated cytokines’ and chemokines’ levels are detected within 0.5–3 h, reaching the peak at 6–12 h and then declining to normal levels at 7–10 days post-inoculation with an AdV vector [98]. Following the intravenous inoculation of an AdV vector, tissue-resident macrophages, including Kupffer cells in the liver and CD169+ and MARCO+ macrophages in the spleen, secrete the proinflammatory cytokine IL1α within 10 min [94]. The activation of IL1α triggers the expression of other proinflammatory cytokines, including IL6 and TNFα, and chemokines CCL2, CXCL1, and CXCL2, within 30 min [100].
The AdV genome can be detected in the endosomes by TLR9, an endosomal sensor that recognizes the double-stranded DNA containing unmethylated CpG motifs and initiates cytokine secretion [101,102,103,104]. Moreover, the stimulation of innate immunity can also be mediated by TLR-independent mechanisms through the DNA-sensing pathway, which induces interferon (IFN) regulatory factor 3 (IRF3) and the NLR family pyrin domain containing 3 (NLRP3) inflammasome, thereby initiating IFN type I production [87,105,106]. Furthermore, the induction of IRF7 occurs following vector escape from endosomes stimulating IFNα/β production in splenic myeloid dendritic cells [107]. The expression of type I IFN is detected within 4 to 6 h after the intravenous administration of HAdV2 [100]. Moreover, the intravenous administration of HAdV2 or HAdV5 results in the initiation of complement pathways and a rapid increase in the complement fragment C3a within 10 min, reaching the peak at 30 min [108]. Complement activation by HAdV2 or HAdV5 does not need antibodies in vivo and they can be activated through both classical and alternative pathways [108]. The natural route of AdV infection may induce a low level of inflammatory response. In contrast, the intravenous administration of a higher number of AdV particles results in robust innate immunity with a high level of several cytokines and chemokines [98]. It may lead to cytokine storm syndrome, hepatotoxicity, disseminated intravascular coagulates, and thrombocytopenia, leading to adverse effects and even death [98,100]. The negative reaction occurs with significantly high vector doses, especially for gene therapy applications [109]. The considerably high amount of vector accumulation predominately in the liver and spleen will initiate an acute inflammatory response due to the induction of high levels of chemokines and cytokines, followed by the recruitment of neutrophils [110,111]. Systemic vector toxicity in the form of significant hepatic necrosis and apoptosis was observed in mice following the intravenous administration of the HAdV5 vector at a high dose (1 × 1010 PFU) [111]. Similarly, tissue damage following the intraportal administration of a nonhuman AdV vector was also noticed in primates [112]. Moreover, the induction of type I IFN following the inoculation of an AdV vector could restrict the duration of transgene expression due to the activation of natural killer (NK) cells [113]. The activated NK cells participate in the rapid elimination of AdV vector-transduced cells, thereby shortening the duration of transgene expression [113,114]. Induction levels of inflammatory cytokines and chemokines may vary with the type of AdV vector, resulting in variability in transgene-specific immunogenicity [115]. For AdV vector-based vaccines, the quality of transgene-specific immune responses is more critical than the duration of transgene expression. However, the level and duration of transgene expression and vector survival are vital for many gene therapy applications.
6. AdV Vector-Mediated Adaptive Immunity
AdV vector-induced innate immunity triggers adaptive immune responses against the vector and the transgene. Following AdV vector inoculation, the APCs transport the vector, vector proteins, or expressed transgene to the draining lymph nodes, where the naïve T cells are primed and differentiate into CD4+ and CD8+ T cells [116]. With the recognition of the viral or transgene epitopes with the MHC class I or II molecules, cellular and humoral immunity is elicited; after this, vector-specific neutralizing antibodies and CD8+ T cells eliminate the vector and vector-infected cells [116,117]. Nevertheless, vector-specific adaptive immunity significantly affects the transgene expression while removing the vector-infected cells, thus posing a challenge to the AdV vector-based gene therapy [118].
The development of anti-AdV neutralizing antibodies (NAbs) contributes towards the inhibition of the vector’s efficacy. The adoptive transfer of HAdV5-specific NAbs to mice negatively impacted the development of transgene-specific immune responses after the administration of an HAdV5 vector-based vaccine [119]. Meanwhile, the depletion of antibodies against the hexon, penton, and fiber significantly improved the transduction efficiency of an AdV vector [120]. The AdV major capsid proteins—hexon, penton, and fiber—are the targets for NAbs against the vector, while the hexon has the predominant reactive sites for NAbs [121]. In human blood samples from the U.S. and developing countries, hexon-specific NAbs titers against HAdV5 were significantly higher than fiber-specific NAbs [122]. The hexon-specific NAbs showed better inhibition of an HAdV5 vector-based vaccine than the fiber-specific NAbs [122]. There are seven hypervariable regions (HVRs) in the flexible loops located at the outer surface of the hexon [123]. HVRs are diverse among AdVs at the amino acid level and contain critical virus-neutralizing epitopes [124]. Anti-fiber Abs can block primary cell receptor interactions, and anti-penton Abs can hinder integrin-mediated internalization [125]. Moreover, the anti-penton Abs showed a synergistic effect with anti-fiber Abs on AdV neutralizing activity and reduced vector-mediated transduction [126,127]. Natural infections with HAdV5 elicit a higher level of fiber-specific NAbs than vaccination with an HAdV5-based vaccine [128]. Different circumstances may present distinct vector-neutralization mechanisms, while the hexon and fiber play vital roles in vector neutralization [121,128]. Therefore, hexon or fiber modifications can circumvent AdV NAbs and serve as one of the strategies to enhance vector efficacy [121,129]. Antibody-mediated AdV neutralization occurs due to virus aggregation, blocking AdV attachment and entry into the host cells [130]. Other mechanisms, such as AdV particle destabilization, abortive virus internalization, and interference of virus uncoating, are also induced by NAbs [125,131,132]. Unique post-entry AdV neutralization by blocking the microtubule-dependent virion translocation with hexon-specific NAbs was also elucidated [125,133].
In addition to AdV NAbs, cell-mediated immunity plays a critical role in AdV clearance [134]. AdV-specific NAbs play a dominant role in virus clearance compared to AdV-specific CD8+ T cells [111]. The AdV-specific CD4+ and CD8+ T cells were detected in healthy adults in peripheral blood mononuclear cells (PBMC) [135,136]. Unlike NAbs, AdV-specific T cell responses are cross-reactive among different AdV types [137]. A CD4 T cell epitope on hexon, H910–924, was identified as a conserved epitope among diverse AdV types [137]. This conserved epitope induces robust T cell proliferation and is recognized by 78% of healthy adults [137,138]. Moreover, four conserved CD8+ T cell epitopes on the hexon can also induce cross-reactive T cell immunity [139].
The durability of humoral and cell-mediated immune responses is critical for the efficacy of AdV vector-based gene delivery [140]. AdV-specific memory CD8 T cells are vital for long-term cell-mediated immunity, which helps in the rapid expansion of these cells in response to the production of antiviral cytokines and cytotoxic molecules following AdV re-infection or vector inoculation [141]. Only a small proportion, around 5–10%, of the activated T cells will become long-lived memory T cells [142]. AdV-specific CD4+ and CD8+ memory T cells are detected in healthy humans from a natural AdV infection [143]. Most AdV-specific CD4+ T cells displayed a central memory-like phenotype, whereas CD8+ T cells exhibited an effector-like phenotype [143]. For AdV vector-based vaccine applications, the immunogen-specific memory T cells were detected in vitro and in vivo [144,145]. These memory T cells perform multiple functions, including degranulation and the production of cytokines [144]. Interestingly, less prevalent HAdVs, such as HAdV26, HAdV35, and HAdV48, showed better CD8+ T cell memory than HAdV5. Enhanced expansion of CD8+ T cells was elicited by using two AdV types for the prime–boost approach [145]. Using more than one AdV vector type will confer better outcomes for vaccine and gene therapy applications compared to multiple doses with the same vector.
7. AdV Tropism, Blood Factors, and Vector Toxicity
While CAR is widely distributed to multiple cell types, the systemic administration of an AdV vector results in predominant tropism to the liver [146]. A biodistribution study in mice inoculated intravenously with an AdV vector demonstrated that the highest amount of transgene expression was detected in the liver [147]. This restricted tropism limits vector distribution to other tissues and leads to liver damage and systemic toxicity [148,149]. The ablation of CAR-binding by modifying the AdV vector did not significantly inhibit biodistribution and hepatotoxicity, implying that other factors besides CAR interaction are vital for liver tropism and hepatotoxicity [150].
Interactions between an AdV vector and the host’s blood factors play a significant role in AdV liver transduction [151]. The modification of the CAR-binding domain on the AdV fiber inhibits the infection of hepatocytes in vitro, but the transfection efficacy of hepatocytes remains unaffected in vivo [151]. This in vivo liver tropism is due to the binding of the blood coagulation factor (F) IX (FIX) and complement factor C4BP to the AdV vector, resulting in interactions with the hepatocellular receptors, including heparan sulfate proteoglycan (HSPG) and low-density lipoprotein receptor-related protein (LRP) for liver transduction [151,152]. The vitamin K-dependent coagulation factors FX, FVII, and protein C can also enhance the hepatocyte transduction of AdVs [153]. In addition, the AdV uptake by Kupffer cells is mediated via the blood factors [151], initiating the production of proinflammatory cytokines such as TNF and IL6, followed by liver damage after the systemic administration of 1 X 1010 transduction units of HAdV5 vector expressing human α1-antitrypsin [154]. The γ-carboxyl glutamic acid (GLA) domain is common among vitamin K-dependent coagulation factors [155]. Unlike the fiber interaction of FIX, the GLA domain of FX can attach to the HVR5 and HVR7 of the hexon in the presence of calcium ions, resulting in efficient transduction of the hepatocytes [97,156,157]. Apart from FX, other coagulation factors showed weak affinity toward the hexon of HAdV5 [109]. This implied that these factors might affect vector tropism through different mechanisms [109,156].
Interactions with coagulation factors may also vary with the AdV type. HAdV31, belonging to species A, interacts with FIX but not with HAdV5, a species C AdV [158]. In comparison, a species D AdV showed weak FX-binding affinity, leading to low hepatic tropism due to a distinct hexon HVR [156]. Understanding receptor binding, coagulation factors’ interactions with AdV, the availability of AdV types, and AdV capsid modifications could help in AdV vector design to lower hepatic tropism and vector toxicity. The fiber shortening or fiber exchange from another AdV type can alter the tropism and significantly decrease the hepatic transduction and toxicity [148]. Mice immunized with an AdV vector, having poly-lysine in the fiber’s knob domain, showed lower levels of IL6 and aspartate aminotransferase (AST), indicating that vector attenuation led to decreased liver toxicity [159]. Replacing the hexon HVR of an AdV vector from a non-FX-binding AdV type can also abolish the coagulation factor-mediated liver transduction [157]. Moreover, nanoparticle coating or polymer encapsulation of AdV vectors effectively decreased liver tropism [160]. Furthermore, advanced AdV retargeting is required to target specific cell types, especially for cancer therapy. A tissue-specific promoter in the E1 region to develop conditional replication-competent AdV vectors, tissue-specific ligand insertion in the fiber knob, and bifunctional adapter molecules are some strategies for targeting specific tissues with AdV vectors [160,161,162].
8. Preexisting Ad Vector Immunity and Its Implications
There is a high prevalence of HAdV infection due to exposure to one or more HAdV types since childhood, due to the availability of over 85 types of HAdVs [163]. Serosurveillance studies showed that 40–90% of humans have NAbs against HAdV5 [163], the most studied HAdV. It seems that children and young adults usually have higher levels of NAbs against AdV than the elderly [19,164]. The development of AdV-specific NAbs following natural infection with one or more AdV types is known as preexisting AdV immunity or preexisting AdV vector immunity, which affects the efficacy of HAdV vectors (Figure 3) [115]. A clinical trial with an HAdV5-based Ebola vaccine showed that volunteers with preexisting AdV vector immunity had a lower response to the vaccine than participants without preexisting AdV immunity [165]. In a clinical trial with the HAdV5-based SARS-CoV-2 vaccine, vaccinees having high preexisting anti-HAdV5 immunity showed lower levels of humoral immune responses compared to participants with low levels of preexisting anti-HAdV5 immunity [166]. Preexisting AdV immunity negatively impacts the vector uptake and development of immunogen-specific adaptive immune responses, thereby affecting the Ad vector’s efficacy [167]. Furthermore, residents of sub-Saharan Africa showed preexisting neutralizing antibodies against some chimpanzee AdVs (ChAdVs), which may restrict the application of ChAdV as an alternative vaccine vector [168].
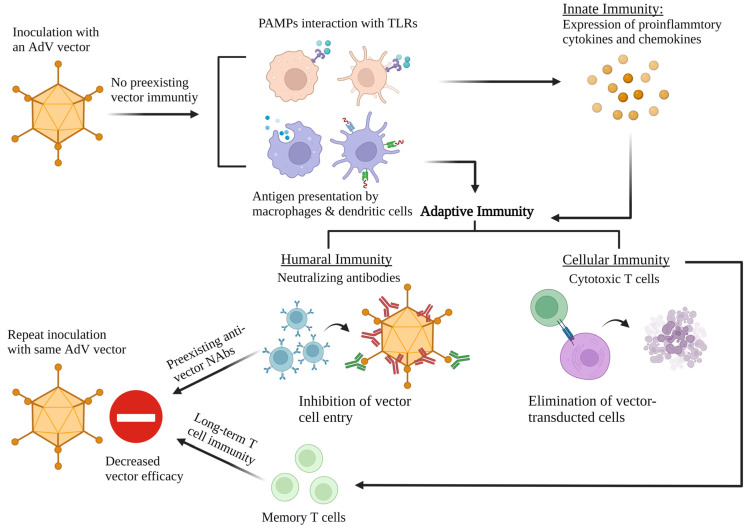
Figure 3.
Activation of innate and adaptive immune responses in response to an adenoviral (AdV) vector. Following inoculation with an AdV vector, activation of innate immunity leads to the expression of proinflammatory chemokines and cytokines. Following antigen expression, processing, and presentation, humoral and cell-mediated immune responses develop. The resultant vector immunity will eliminate the vector, leading to a reduced duration of transgene expression. The development of vector-specific neutralizing antibodies (NAbs) and memory T cells provide long-term vector immunity and suppress subsequent inoculation with the same AdV vector. PAMP, pathogen-associated molecular patterns; TLRs, Toll-like receptors.
In addition to preexisting AdV immunity, the first inoculation with an AdV vector will also lead to vector-specific immune responses, impacting the subsequent administration of the same vector [20]. To evaluate the durability of vector immunity and its effect on the development of immunogen-specific immune responses, naïve or HAdV5-primed mice were immunized with the HAdV-H5HA (HAdV5 vector expressing H5N1 hemagglutinin (HA)) at 1, 3, 6, and 10 months after priming and challenged with an antigenically distinct H5N1 influenza virus four weeks following vaccination [169]. With time, there was a continuous decline in vector immunity, with a constant improvement in HA-specific immune responses and enhanced protection against a heterologous H5N1 virus [169]. The drop in vector immunity at six months and onwards was sufficient for inducing the required level of protective immunity. This study implies that vector immunity can inhibit vector efficacy following repeated vaccination with the same vector. Moreover, annual immunization with the same vector should be possible due to the decline in vector immunity to levels that may not impact the vector’s efficacy significantly. The prime–boost approach, using different AdV type vectors, is an excellent strategy to substantially improve the effectiveness of the AdV vector platform by eluding the vector-specific immunity during the boosting step [170]. This approach was used for the HAdV5/HAdV26-based prime–boost vaccine for SARS-CoV-2 [171]. An HAdV11 vector, which showed better transduction efficiency in smooth muscle cells, synoviocytes, dendritic cells, and cardiovascular tissues compared to HAdV5, was not hampered by preexisting HAdV5-specific NAbs and was investigated as a vector for gene therapy [74].
9. Strategies to Circumvent Preexisting Vector Immunity
HAdVs with low seroprevalence in humans, including HAdV11, 35, or 50 of species B, and HAdV26, 48, or 49 of species D, are unaffected by preexisting AdV immunity [172,173,174]. In particular, the HAdV35 and HAdV26 vectors were evaluated in humans for their vaccine efficacy against Mycobacterium tuberculosis, malaria, HIV, Ebola virus, and SARS-CoV-2 [175,176,177,178,179,180]. For gene therapy, a HAdV35 vector demonstrated better transduction capability towards glioma cells, indicating its utility in treating malignant glioma [181]. However, six rare HAdVs (HAdV11, HAdV26, HAdV35, HAdV48, HAdV49, and HAdV50) showed lower immunogenicity than the HAdV5 vector [174]. HAdV35- or HAdV26-based Ebola vaccines induced lower immunogenicity and protection against the Ebola virus in nonhuman primates compared to the HAdV5-based vaccine [115]. Since the AdV-specific cellular immunity is comparatively cross-reactive, HAdV26-specific cellular immune responses at a baseline were detected in humans without HAdV26-specific NAbs [182]. The HAdV35-specific CD4+ T cells were detected in individuals without HAdV35-specific NAbs [135]. These studies suggest that AdV-specific T lymphocytes are cross-reactive among multiple AdV types. On the other hand, HAdV35, HAdV26, and HAdV48 interact with CD46 as the primary cellular receptor and induce enhanced innate immunity compared to HAdV5 [183]. The rare HAdVs offer attractive alternatives to avoid preexisting vector immunity for efficient transgene expression. Several strategies that are utilized to circumvent the preexisting vector immunity are outlined below (Figure 4).
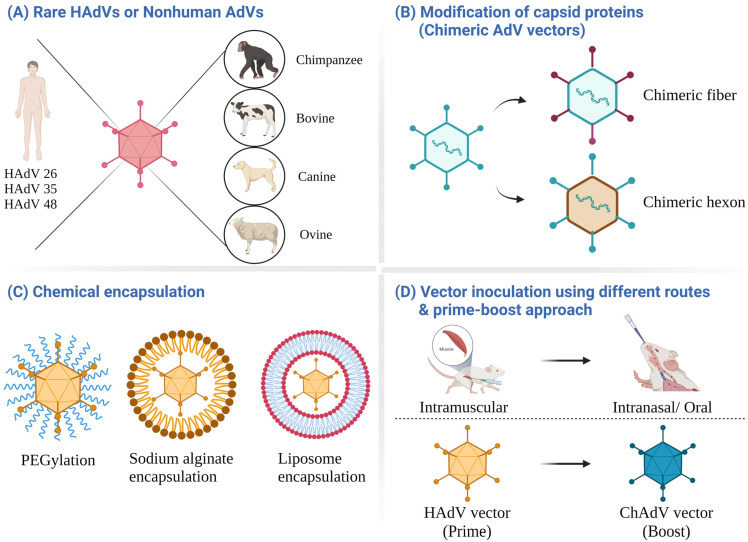
Figure 4.
Circumventing strategies against preexisting vector immunity. (A) Inoculation with a rare human adenoviral (HAdV) or nonhuman AdV can evade the preexisting vector immunity. (B) Since most of the neutralizing antibodies (NAbs) target the capsid proteins, such as the fiber and hexon, exchanging the capsid and/or fiber proteins with a different AdV type can elude the preexisting vector immunity. (C) Chemical encapsulation can mask the AdV vector from antibody-mediated neutralization. (D) The use of different routes of inoculation or different vector platforms (prime–boost approach) can circumvent the preexisting vector immunity. ChAdV, chimpanzee adenovirus; PEG, polyethylene glycol.
Alternatively, nonhuman AdV vectors of simian, bovine, porcine, ovine, canine, or avian origin were developed to evade the AdVs’ preexisting immunity since AdVs are species-specific and do not naturally infect humans [184]. Cross-NAbs against ChAdV are rarely detected in developed countries’ populations but showed higher prevalence in sub-Saharan Africa [185]. Replication-defective ChAdV vectors can grow efficiently in the cell lines that support the replication of HAdV5 vectors, e.g., the HEK 293 cell line [186]. In addition, ChAd vectors can elicit high levels of humoral and cellular immune responses against the immunogen, similar to the HAdV5 vector expressing the same immunogen, and the immunogenicity is not significantly inhibited in the presence of preexisting HAdV immunity [187,188]. However, there is variability in the resultant immunogenicity with different ChAdV types [189]. ChAdV vector vaccines against SARS-CoV-2, Ebola virus, malaria, HIV, and respiratory syncytial virus have been evaluated for their efficacy in humans [190,191,192,193]. AstraZeneca’s SARS-CoV-2 vaccine based on the ChAdV vector platform (ChAdOX1) was licensed in several countries [180]. The bovine Ad (BAdV) type 3 (BAdV3) belonging to the Mastadenovirus genus was generated as a gene delivery vector [194] and showed excellent potential in overcoming preexisting vector immunity [195]. Preexisting HAdV NAbs do not cross-react with BAdV3 or hamper the transduction ability of the BAdV3 vector [195]. The HAdV-specific cellular immunity showed minimal cross-reactivity with the BAdV3 vector [196]. Moreover, the BAdV3 vector can efficiently transduce various tissues, with relatively prolonged persistence compared to the HAdV5 vector [197]. Better stimulation of innate immunity with higher expression of proinflammatory chemokines and cytokines was also shown in mice inoculated with a BAdV vector compared to that of a HAdV5 vector [198]. A BAdV3 vector-based influenza vaccine provided better immunogenicity and protection than the HAdV5 vector-based influenza vaccine [199]. Since BAdV3 utilizes sialic acid as the receptor for virus entry [55], this vector platform is suitable for nasal delivery [200]. It seems that there is enhanced antigen processing through the autophagy pathway in mice immunized with a BAdV3 vector-based vaccine compared to the HAdV5 vector-based vaccine [200]. Overall, the BAdV3 vectors circumvent the preexisting vector immunity and confer robust immunogenicity. Other nonhuman AdV vectors, such as porcine AdV, ovine AdV, canine AdV, and avian AdV, are also under evaluation as alternative vectors for gene delivery [187].
Since the capsid proteins are the main targets of NAbs, modification of the capsid proteins is a relevant strategy to overcome preexisting vector immunity. The HVR sequence of the HAdV5 hexon was exchanged with the corresponding regions from HAdV48 to design a hexon-chimeric AdV vector [201]. This vector showed robust immunogenicity in the presence of high levels of preexisting anti-HAdV5 NAbs [201]. Similarly, fiber-chimeric AdV vectors were generated by exchanging the knob domain of HAdV5 with the knob region of HAdV3 [129]. This also enhanced its ability to circumvent preexisting HAdV5 immunity in mice pre-immunized with the unmodified HAdV5 vector [129]. There was increased transduction efficiency of a chimeric HAdV5 vector with the HAdV35 fiber in HAdV5-immunized mice [202]. The same strategy can also be extended to nonhuman AdVs. A chimeric HAdV5 vector with the BAdV4 fiber demonstrated reduced innate immunity and interactions with blood coagulation factors in mice compared with the HAdV5 vector [203]. These modifications were also used for gene therapy applications to redirect the AdV tropism to the targeted cells [204,205].
Chemical encapsulation can shield the antigenic epitopes on the AdV surface and thus help to evade the AdV NAbs. PEGylation of the AdV vector can retain the virus transduction capability but protect the vector from NAbs [206]. The transgene expression with the PEG-conjugated AdV vector was higher than that with the untreated vector in mice preimmunized with the unmodified AdV vector [207]. Moreover, the PEGylation of AdV vectors reduces the levels of vector-induced innate immunity and thereby decreases vector toxicity, such as thrombocytopenia or increased levels of ALT [207]. PEGylation with PLGA encapsulation also enhanced vector stability and gene transfection efficiency in vitro [208]. Moreover, encapsulation of AdV vectors with liposomes can also provide resistance to AdV NAbs [209], enhancing the transduction efficiency with repeated vector administration [210]. It can also reduce hepatic uptake and improve the transduction of CAR-deficient cells [211,212]. PEGylation in liposome-encapsulated AdV vectors can further reduce cytotoxicity, hemolytic activity, anti-vector immunity, and innate immunity [213]. Moreover, the microencapsulation of AdVs into biodegradable sodium alginate microspheres also eluded vector-specific immunity [20].
Sequential inoculations with different routes are an alternative strategy to conquer the anti-vector immunity during repeat administration. It was demonstrated that the preexisting immunity from the intramuscular injection of an AdV vector does not significantly affect the immunogenicity of the same AdV-based vaccine when administrated intranasally [214]. Similarly, mice pre-immunized with HAdV5 intramuscularly or intranasally do not show decreased humoral immunity to the transgene from subsequent HAdV vector oral administration [215]. The intranasal administration of AdVs infects localized antigen-presenting cells that carry the antigen into the local draining lymph node. This causes most activated effector T cells to be localized in the respiratory system, and long-living memory T cells remain retained within these tissues [91]. Parenteral administration usually circulates the effector T cells into systemic lymphoid organs, with minimal memory T cells in the respiratory tract [91].
10. Conclusions and Future Directions
AdV vectors have been at the forefront of promising gene delivery platforms for vaccines and gene therapy. The emergency-use authorization of three AdV vector-based SARS-CoV-2 vaccines is one of the AdV vector platform’s success stories. This platform has extensively been utilized as a gene delivery system for vaccine or gene therapy applications in several human clinical trials. With our advancing understanding of AdV biology, vector design has versatility depending on the need. However, the preexisting vector immunity and induction of enhanced innate immunity at higher vector doses are notable limitations that could significantly affect the efficacy of the AdV vector platform. The development of rare human and nonhuman AdVs as gene delivery platforms has helped to overcome the preexisting vector immunity issue. The concept of a prime–boost approach with two different AdV vector types, or priming with an AdV vector followed by a boost with another vector system, could significantly reduce the adverse impact of innate immunity.
Additional work is needed to design strategies for further controlling vector tropism to enhance its effectiveness while significantly inhibiting hepatic toxicity, especially for gene therapy applications. Activation of innate immunity by AdV vectors is a unique strength, vital for the success of AdV vector-based vaccines, by eliciting enhanced immunogen-specific immune responses. Therefore, the induction of innate immunity needs to be better managed to avoid adverse effects without compromising its adjuvant impact. AdV vectors for mucosal immunization require further exploration to provide better vaccine efficacy against mucosal pathogens. Investigation of other rare human or nonhuman AdVs for vector development should continue to expand the vector choices for various gene therapy applications.
Further investigations to determine strategies to inhibit the AdV vector binding to the blood factors will be critical for improving the vector’s versatility. The worth of the same or different AdV vector for annual vaccination needs to be explored. New research in nanoparticles and AdV capsid modifications will further boost the quality and efficacy of AdV vector-based gene delivery applications. Additional work is necessary to further enhance the durability of immunogen-specific immunity and the duration of transgene expression when delivered through an AdV vector.
Acknowledgments
We thank all the researchers whose work is referenced in this review article. We apologize to other researchers whose work we failed to mention due to oversight on our part.
Author Contributions
W.-C.W. and E.E.S. wrote the manuscript and designed the figures; S.K.M. finalized the concept and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Public Health Service (PHS) grants AI059374 and AI158177 from the National Institute of Allergy and Infectious Diseases (NIAID) and the USDA National Institute of Food and Agriculture [Hatch #1014146].
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hllleman M.R., Werner J.H. Recovery of New Agent from Patients with Acute Respiratory Illness. Exp. Biol. Med. 1954;85:183–188. doi: 10.3181/00379727-85-20825. [DOI] [PubMed] [Google Scholar]
- 2.Robinson C.M., Seto D., Jones M.S., Dyer D.W., Chodosh J. Molecular evolution of human species D adenoviruses. Infect. Genet. Evol. 2011;11:1208–1217. doi: 10.1016/j.meegid.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benkő M., Aoki K., Arnberg N., Davison A.J., Echavarría M., Hess M., Jones M.S., Kaján G.L., Kajon A.E., Mittal S.K., et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J. Gen. Virol. 2022;103:001721. doi: 10.1099/jgv.0.001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Human Adenovirus Working Group. [(accessed on 23 April 2020)]. Available online: http://hadvwg.gmu.edu/
- 5.Wang Y., Huang S. Adenovirus technology for gene manipulation and functional studies. Drug Discov. Today. 2000;5:10–16. doi: 10.1016/S1359-6446(99)01433-6. [DOI] [PubMed] [Google Scholar]
- 6.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozarsky K.F., Wilson J. Gene therapy: Adenovirus vectors. Curr. Opin. Genet. Dev. 1993;3:499–503. doi: 10.1016/0959-437X(93)90126-A. [DOI] [PubMed] [Google Scholar]
- 9.Balagué C., Zhou J., Dai Y., Alemany R., Josephs S.F., Andreason G., Hariharan M., Sethi E., Prokopenko E., Jan H.-Y., et al. Sustained high-level expression of full-length human factor VIII and restoration of clotting activity in hemophilic mice using a minimal adenovirus vector. Blood. 2000;95:820–828. doi: 10.1182/blood.V95.3.820.003k32_820_828. [DOI] [PubMed] [Google Scholar]
- 10.Ehrhardt A., Kay M.A. A new adenoviral helper–dependent vector results in long-term therapeutic levels of human coagulation factor IX at low doses in vivo. Blood. 2002;99:3923–3930. doi: 10.1182/blood.V99.11.3923. [DOI] [PubMed] [Google Scholar]
- 11.VanderVeen N., Raja N., Yi E., Appelman H., Ng P., Palmer D., Zamler D., Dzaman M., Lowenstein P.R., Castro M.G. Preclinical Efficacy and Safety Profile of Allometrically Scaled Doses of Doxycycline Used to Turn “On” Therapeutic Transgene Expression from High-Capacity Adenoviral Vectors in a Glioma Model. Hum. Gene Ther. Methods. 2016;27:98–111. doi: 10.1089/hgtb.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricobaraza A., Gonzalez-Aparicio M., Mora-Jimenez L., Lumbreras S., Hernandez-Alcoceba R. High-Capacity Adenoviral Vectors: Expanding the Scope of Gene Therapy. Int. J. Mol. Sci. 2020;21:3643. doi: 10.3390/ijms21103643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw A.R., Suzuki M. Immunology of Adenoviral Vectors in Cancer Therapy. Mol. Ther.—Methods Clin. Dev. 2019;15:418–429. doi: 10.1016/j.omtm.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crenshaw B.J., Jones L.B., Bell C.R., Kumar S., Matthews Q.L. Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy. Biomedicines. 2019;7:61. doi: 10.3390/biomedicines7030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Martin N., Ramani S.R., Hackney J.A., Tom I., Wranik B.J., Chan M., Wu J., Paluch M.T., Takeda K., Hass P.E., et al. The extracellular interactome of the human adenovirus family reveals diverse strategies for immunomodulation. Nat. Commun. 2016;7:11473. doi: 10.1038/ncomms11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi J.-W., Lee J.-S., Kim S.W., Yun C.-O. Evolution of oncolytic adenovirus for cancer treatment. Adv. Drug Deliv. Rev. 2012;64:720–729. doi: 10.1016/j.addr.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Kim E., Kim J.-H., Shin H.-Y., Lee H., Yang J.M., Kim J., Sohn J.-H., Kim H., Yun C.-O. Ad-mTERT-Δ19, a Conditional Replication-Competent Adenovirus Driven by the Human Telomerase Promoter, Selectively Replicates in and Elicits Cytopathic Effect in a Cancer Cell-Specific Manner. Hum. Gene Ther. 2003;14:1415–1428. doi: 10.1089/104303403769211637. [DOI] [PubMed] [Google Scholar]
- 18.Chandler R.J., Venditti C.P. Gene therapy for metabolic diseases. Transl. Sci. Rare Dis. 2016;1:73–89. doi: 10.3233/TRD-160007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dharmapuri S., Peruzzi D., Aurisicchio L. Engineered adenovirus serotypes for overcoming anti-vector immunity. Expert Opin. Biol. Ther. 2009;9:1279–1287. doi: 10.1517/14712590903187053. [DOI] [PubMed] [Google Scholar]
- 20.Sailaja G., HogenEsch H., North A., Hays J., Mittal S.K. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002;9:1722–1729. doi: 10.1038/sj.gt.3301858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charman M., Herrmann C., Weitzman M.D. Viral and cellular interactions during adenovirus DNA replication. FEBS Lett. 2019;593:3531–3550. doi: 10.1002/1873-3468.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sayedahmed E.E., Elkashif A., Alhashimi M., Sambhara S., Mittal S.K. Adenoviral Vector-Based Vaccine Platforms for Developing the Next Generation of Influenza Vaccines. Vaccines. 2020;8:574. doi: 10.3390/vaccines8040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahi Y.S., Mittal S.K. Components of Adenovirus Genome Packaging. Front. Microbiol. 2016;7:1503. doi: 10.3389/fmicb.2016.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha B., Wong C.M., Parks R.J. The Adenovirus Genome Contributes to the Structural Stability of the Virion. Viruses. 2014;6:3563–3583. doi: 10.3390/v6093563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saha B., Parks R.J. Human adenovirus type 5 vectors deleted of early region 1 (E1) undergo limited expression of early replicative E2 proteins and DNA replication in non-permissive cells. PLoS ONE. 2017;12:e0181012. doi: 10.1371/journal.pone.0181012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz M.S. Function of adenovirus E3 proteins and their interactions with immunoregulatory cell proteins. J. Gene Med. 2004;6:S172–S183. doi: 10.1002/jgm.495. [DOI] [PubMed] [Google Scholar]
- 27.Reddy V.S., Nemerow G.R. Structures and organization of adenovirus cement proteins provide insights into the role of capsid maturation in virus entry and infection. Proc. Natl. Acad. Sci. USA. 2014;111:11715. doi: 10.1073/pnas.1408462111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart P.L. Adenoviral Vectors for Gene Therapy. 2nd ed. Academic Press; San Diego, CA, USA: 2016. pp. 1–26. [Google Scholar]
- 29.Greber U.F., Flatt J.W. Adenovirus Entry: From Infection to Immunity. Annu. Rev. Virol. 2019;6:177–197. doi: 10.1146/annurev-virology-092818-015550. [DOI] [PubMed] [Google Scholar]
- 30.Bi Y., Tan S., Yang Y., Wong G., Zhao M., Zhang Q., Wang Q., Zhao X., Li L., Yuan J., et al. Clinical and Immunological Characteristics of Human Infections With H5N6 Avian Influenza Virus. Clin. Infect. Dis. 2018;68:1100–1109. doi: 10.1093/cid/ciy681. [DOI] [PubMed] [Google Scholar]
- 31.Pichla-Gollon S.L., Drinker M., Zhou X., Xue F., Rux J.J., Gao G.-P., Wilson J.M., Ertl H.C.J., Burnett R.M., Bergelson J.M. Structure-Based Identification of a Major Neutralizing Site in an Adenovirus Hexon. J. Virol. 2007;81:1680–1689. doi: 10.1128/JVI.02023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vellinga J., Van Der Heijdt S., Hoeben R. The adenovirus capsid: Major progress in minor proteins. J. Gen. Virol. 2005;86:1581–1588. doi: 10.1099/vir.0.80877-0. [DOI] [PubMed] [Google Scholar]
- 33.Gallardo J., Pérez-Illana M., Martín-González N., Martín C.S. Adenovirus Structure: What Is New? Int. J. Mol. Sci. 2021;22:5240. doi: 10.3390/ijms22105240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai X., Wu L., Sun R., Zhou Z.H. Atomic Structures of Minor Proteins VI and VII in Human Adenovirus. J. Virol. 2017;91:e00850-17. doi: 10.1128/JVI.00850-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulanayake S., Tikoo S.K. Adenovirus Core Proteins: Structure and Function. Viruses. 2021;13:388. doi: 10.3390/v13030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elkashif A., Alhashimi M., E Sayedahmed E., Sambhara S., Mittal S.K. Adenoviral vector-based platforms for developing effective vaccines to combat respiratory viral infections. Clin. Transl. Immunol. 2021;10:e1345. doi: 10.1002/cti2.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones M.S., Harrach B., Ganac R.D., Gozum M.M.A., Delacruz W., Riedel B., Pan C., Delwart E., Schnurr D.P. New Adenovirus Species Found in a Patient Presenting with Gastroenteritis. J. Virol. 2007;81:5978–5984. doi: 10.1128/JVI.02650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergelson J.M. Receptors mediating adenovirus attachment and internalization. Biochem. Pharmacol. 1999;57:975–979. doi: 10.1016/S0006-2952(98)00332-3. [DOI] [PubMed] [Google Scholar]
- 39.Campos S.K., Barry M.A. Current Advances and Future Challenges in Adenoviral Vector Biology and Targeting. Curr. Gene Ther. 2007;7:189–204. doi: 10.2174/156652307780859062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nemerow G.R. Cell Receptors Involved in Adenovirus Entry. Virology. 2000;274:1–4. doi: 10.1006/viro.2000.0468. [DOI] [PubMed] [Google Scholar]
- 41.Wang X., Bergelson J.M. Coxsackievirus and Adenovirus Receptor Cytoplasmic and Transmembrane Domains Are Not Essential for Coxsackievirus and Adenovirus Infection. J. Virol. 1999;73:2559–2562. doi: 10.1128/JVI.73.3.2559-2562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stasiak A.C., Stehle T. Human adenovirus binding to host cell receptors: A structural view. Med. Microbiol. Immunol. 2019;209:325–333. doi: 10.1007/s00430-019-00645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergelson J.M., Cunningham J.A., Droguett G., Kurt-Jones E.A., Krithivas A., Hong J.S., Horwitz M.S., Crowell R.L., Finberg R.W. Isolation of a Common Receptor for Coxsackie B Viruses and Adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 44.Tomko R.P., Xu R., Philipson L. HCAR and MCAR: The human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roelvink P.W., Lizonova A., Lee J.G.M., Li Y., Bergelson J.M., Finberg R.W., Brough D.E., Kovesdi I., Wickham T.J. The Coxsackievirus-Adenovirus Receptor Protein Can Function as a Cellular Attachment Protein for Adenovirus Serotypes from Subgroups A, C, D, E, and F. J. Virol. 1998;72:7909–7915. doi: 10.1128/JVI.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Short J.J., Pereboev A.V., Kawakami Y., Vasu C., Holterman M.J., Curiel D.T. Adenovirus serotype 3 utilizes CD80 (B7.1) and CD86 (B7.2) as cellular attachment receptors. Virology. 2004;322:349–359. doi: 10.1016/j.virol.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 47.Marttila M., Persson D., Gustafsson D., Liszewski M.K., Atkinson J.P., Wadell G., Arnberg N. CD46 Is a Cellular Receptor for All Species B Adenoviruses except Types 3 and 7. J. Virol. 2005;79:14429–14436. doi: 10.1128/JVI.79.22.14429-14436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuve S., Wang H., Ware C., Liu Y., Gaggar A., Bernt K., Shayakhmetov D., Li Z., Strauss R., Stone D., et al. A New Group B Adenovirus Receptor Is Expressed at High Levels on Human Stem and Tumor Cells. J. Virol. 2006;80:12109–12120. doi: 10.1128/JVI.01370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fleischli C., Sirena D., Lesage G., Havenga M.J.E., Cattaneo R., Greber U.F., Hemmi S. Species B adenovirus serotypes 3, 7, 11 and 35 share similar binding sites on the membrane cofactor protein CD46 receptor. J. Gen. Virol. 2007;88:2925–2934. doi: 10.1099/vir.0.83142-0. [DOI] [PubMed] [Google Scholar]
- 50.Persson B.D., Reiter D.M., Marttila M., Mei Y.-F., Casasnovas J.M., Arnberg N., Stehle T. Adenovirus type 11 binding alters the conformation of its receptor CD46. Nat. Struct. Mol. Biol. 2007;14:164–166. doi: 10.1038/nsmb1190. [DOI] [PubMed] [Google Scholar]
- 51.Wang H., Li Z.-Y., Liu Y., Persson J., Beyer I., Möller T., Koyuncu D., Drescher M.R., Strauss R., Zhang X.-B., et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 2010;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nilsson E.C., Storm R.J., Bauer J., Johansson S.M.C., Lookene A., Ångström J., Hedenström M., Eriksson T.L., Frängsmyr L., Rinaldi S., et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat. Med. 2010;17:105–109. doi: 10.1038/nm.2267. [DOI] [PubMed] [Google Scholar]
- 53.Arnberg N., Edlund K., Kidd A.H., Wadell G. Adenovirus Type 37 Uses Sialic Acid as a Cellular Receptor. J. Virol. 2000;74:42–48. doi: 10.1128/JVI.74.1.42-48.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lenman A., Liaci A.M., Liu Y., Frängsmyr L., Frank M., Blaum B.S., Chai W., Podgorski I.I., Harrach B., Benkő M., et al. Polysialic acid is a cellular receptor for human adenovirus 52. Proc. Natl. Acad. Sci. USA. 2018;115:E4264–E4273. doi: 10.1073/pnas.1716900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X., Bangari D.S., Sharma A., Mittal S.K. Bovine adenovirus serotype 3 utilizes sialic acid as a cellular receptor for virus entry. Virology. 2009;392:162–168. doi: 10.1016/j.virol.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma A., Li X., Bangari D.S., Mittal S.K. Adenovirus receptors and their implications in gene delivery. Virus Res. 2009;143:184–194. doi: 10.1016/j.virusres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bangari D.S., Shukla S., Mittal S.K. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem. Biophys. Res. Commun. 2005;327:960–966. doi: 10.1016/j.bbrc.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 58.Bangari D.S., Mittal S.K. Porcine adenovirus serotype 3 internalization is independent of CAR and αvβ3 or αvβ5 integrin. Virology. 2005;332:157–166. doi: 10.1016/j.virol.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Nemerow G.R., Stewart P.L. Insights into Adenovirus Uncoating from Interactions with Integrins and Mediators of Host Immunity. Viruses. 2016;8:337. doi: 10.3390/v8120337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stewart P.L., Nemerow G.R. Cell integrins: Commonly used receptors for diverse viral pathogens. Trends Microbiol. 2007;15:500–507. doi: 10.1016/j.tim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 61.Nemerow G., Pache L., Reddy V., Stewart P. Insights into adenovirus host cell interactions from structural studies. Virology. 2009;384:380–388. doi: 10.1016/j.virol.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Medina-Kauwe L.K. Endocytosis of adenovirus and adenovirus capsid proteins. Adv. Drug Deliv. Rev. 2003;55:1485–1496. doi: 10.1016/j.addr.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Meier O., Greber U.F. Adenovirus endocytosis. J. Gene Med. 2004;6:S152–S163. doi: 10.1002/jgm.553. [DOI] [PubMed] [Google Scholar]
- 64.Wiethoff C.M., Wodrich H., Gerace L., Nemerow G.R. Adenovirus Protein VI Mediates Membrane Disruption following Capsid Disassembly. J. Virol. 2005;79:1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greber U.F., Suomalainen M., Stidwill R.P., Boucke K., Ebersold M.W., Helenius A. The role of the nuclear pore complex in adenovirus DNA entry. EMBO J. 1997;16:5998–6007. doi: 10.1093/emboj/16.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suomalainen M., Nakano M.Y., Keller S., Boucke K., Stidwill R.P., Greber U.F. Microtubule-dependent Plus- and Minus End–directed Motilities Are Competing Processes for Nuclear Targeting of Adenovirus. J. Cell Biol. 1999;144:657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang J. Adenovirus Vectors: Excellent Tools for Vaccine Development. Immune Netw. 2021;21:e2. doi: 10.4110/in.2021.21.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lasaro O.M., Ertl H.C. New Insights on Adenovirus as Vaccine Vectors. Mol. Ther. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ng P., Graham F.L. Construction of First-Generation Adenoviral Vectors. Methods Mol. Med. 2002;69:389–414. doi: 10.1385/1-59259-141-8:389. [DOI] [PubMed] [Google Scholar]
- 70.Graham F.L., Smiley J., Russell W.C., Nairn R. Characteristics of a Human Cell Line Transformed by DNA from Human Adenovirus Type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 71.Kovesdi I., Hedley S.J. Adenoviral Producer Cells. Viruses. 2010;2:1681–1703. doi: 10.3390/v2081681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sayedahmed E.E., Kumari R., Mittal S.K. Current Use of Adenovirus Vectors and Their Production Methods. Methods Mol. Biol. 2019;1937:155–175. doi: 10.1007/978-1-4939-9065-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parks R., Cummings D., Evelegh C., Sankar U., Graham F. A High-Efficiency Cre/loxP-Based System for Construction of Adenoviral Vectors. Hum. Gene Ther. 1999;10:2667–2672. doi: 10.1089/10430349950016708. [DOI] [PubMed] [Google Scholar]
- 74.Holterman L., Vogels R., van der Vlugt R., Sieuwerts M., Grimbergen J., Kaspers J., Geelen E., van der Helm E., Lemckert A., Gillissen G., et al. Novel Replication-Incompetent Vector Derived from Adenovirus Type 11 (Ad11) for Vaccination and Gene Therapy: Low Seroprevalence and Non-Cross-Reactivity with Ad5. J. Virol. 2004;78:13207–13215. doi: 10.1128/JVI.78.23.13207-13215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y., A Nunes F., Berencsi K., E Furth E., Gönczöl E., Wilson J.M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Q., Finer M.H. Second–generation adenovirus vectors. Nat. Med. 1996;2:714–716. doi: 10.1038/nm0696-714. [DOI] [PubMed] [Google Scholar]
- 77.Alba R., Bosch A., Chillon M. Gutless adenovirus: Last-generation adenovirus for gene therapy. Gene Ther. 2005;12:S18–S27. doi: 10.1038/sj.gt.3302612. [DOI] [PubMed] [Google Scholar]
- 78.Brescia M., Janssen J.M., Liu J., Gonçalves M.A.F.V. High-Capacity Adenoviral Vectors Permit Robust and Versatile Testing of DMD Gene Repair Tools and Strategies in Human Cells. Cells. 2020;9:869. doi: 10.3390/cells9040869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parks R.J., Chen L., Anton M., Sankar U., Rudnicki M.A., Graham F.L. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sargent K.L., Ng P., Evelegh C., Graham F.L., Parks R.J. Development of a size-restricted pIX-deleted helper virus for amplification of helper-dependent adenovirus vectors. Gene Ther. 2004;11:504–511. doi: 10.1038/sj.gt.3302107. [DOI] [PubMed] [Google Scholar]
- 81.Mantwill K., Klein F.G., Wang D., Hindupur S.V., Ehrenfeld M., Holm P.S., Nawroth R. Concepts in Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2021;22:10522. doi: 10.3390/ijms221910522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao Y., Liu Z., Li L., Wu J., Zhang H., Zhang H., Lei T., Xu B. Oncolytic Adenovirus: Prospects for Cancer Immunotherapy. Front. Microbiol. 2021;12:707290. doi: 10.3389/fmicb.2021.707290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knowles M.K., Roberts D., Craig S., Sheen M., Nadin-Davis S.A., Wandeler A.I. In vitro and in vivo genetic stability studies of a human adenovirus type 5 recombinant rabies glycoprotein vaccine (ONRAB) Vaccine. 2009;27:2662–2668. doi: 10.1016/j.vaccine.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 84.Jager L., Ehrhardt A. Emerging adenoviral vectors for stable correction of genetic disorders. Curr. Gene Ther. 2007;7:272–283. doi: 10.2174/156652307781369074. [DOI] [PubMed] [Google Scholar]
- 85.Pichlmair A., e Sousa C.R. Innate Recognition of Viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 86.Rhee E.G., Blattman J.N., Kasturi S.P., Kelley R.P., Kaufman D.R., Lynch D.M., La Porte A., Simmons N.L., Clark S.L., Pulendran B., et al. Multiple Innate Immune Pathways Contribute to the Immunogenicity of Recombinant Adenovirus Vaccine Vectors. J. Virol. 2011;85:315–323. doi: 10.1128/JVI.01597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu J., Huang X., Yang Y. Innate Immune Response to Adenoviral Vectors Is Mediated by both Toll-Like Receptor-Dependent and -Independent Pathways. J. Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yeh P., Perricaudet M. Advances in adenoviral vectors: From genetic engineering to their biology. FASEB J. 1997;11:615–623. doi: 10.1096/fasebj.11.8.9240963. [DOI] [PubMed] [Google Scholar]
- 89.Deal C., Pekosz A., Ketner G. Prospects for oral replicating adenovirus-vectored vaccines. Vaccine. 2013;31:3236–3243. doi: 10.1016/j.vaccine.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Q., Su X., Seto D., Zheng B.-J., Tian X., Sheng H., Li H., Wang Y., Zhou R. Construction and characterization of a replication-competent human adenovirus type 3-based vector as a live-vaccine candidate and a viral delivery vector. Vaccine. 2009;27:1145–1153. doi: 10.1016/j.vaccine.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 91.Santosuosso M., McCormick S., Xing Z. Adenoviral Vectors for Mucosal Vaccination Against Infectious Diseases. Viral Immunol. 2005;18:283–291. doi: 10.1089/vim.2005.18.283. [DOI] [PubMed] [Google Scholar]
- 92.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 93.Kawasaki T., Kawai T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Paolo N.C., Miao E.A., Iwakura Y., Murali-Krishna K., Aderem A., Flavell R.A., Papayannopoulou T., Shayakhmetov D.M. Virus Binding to a Plasma Membrane Receptor Triggers Interleukin-1α-Mediated Proinflammatory Macrophage Response In Vivo. Immunity. 2009;31:110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thaci B., Ulasov I.V., Wainwright D.A., Lesniak M.S. The Challenge for Gene Therapy: Innate Immune Response to Adenoviruses. Oncotarget. 2011;2:113–121. doi: 10.18632/oncotarget.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Atasheva S., Shayakhmetov D.M. Adenovirus sensing by the immune system. Curr. Opin. Virol. 2016;21:109–113. doi: 10.1016/j.coviro.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Doronin K., Flatt J.W., Di Paolo N.C., Khare R., Kalyuzhniy O., Acchione M., Sumida J.P., Ohto U., Shimizu T., Akashi-Takamura S., et al. Coagulation Factor X Activates Innate Immunity to Human Species C Adenovirus. Science. 2012;338:795–798. doi: 10.1126/science.1226625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Atasheva S., Shayakhmetov D.M. Cytokine Responses to Adenovirus and Adenovirus Vectors. Viruses. 2022;14:888. doi: 10.3390/v14050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Atasheva S., Yao J., Shayakhmetov D.M. Innate immunity to adenovirus: Lessons from mice. FEBS Lett. 2019;593:3461–3483. doi: 10.1002/1873-3468.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cerullo V., Seiler M.P., Mane V., Brunetti-Pierri N., Clarke C., Bertin T.K., Rodgers J.R., Lee B. Toll-like Receptor 9 Triggers an Innate Immune Response to Helper-dependent Adenoviral Vectors. Mol. Ther. 2007;15:378–385. doi: 10.1038/sj.mt.6300031. [DOI] [PubMed] [Google Scholar]
- 102.Barlan A.U., Griffin T.M., Mcguire K.A., Wiethoff C.M. Adenovirus Membrane Penetration Activates the NLRP3 Inflammasome. J. Virol. 2011;85:146–155. doi: 10.1128/JVI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Teigler J.E., Kagan J.C., Barouch D.H. Late Endosomal Trafficking of Alternative Serotype Adenovirus Vaccine Vectors Augments Antiviral Innate Immunity. J. Virol. 2014;88:10354–10363. doi: 10.1128/JVI.00936-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Skountzou I., Brock N., Lelutiu N., Compans R.W. Immunopotentiators in Modern Vaccines. 2nd ed. Academic Press; San Diego, CA, USA: 2017. pp. 399–419. [Google Scholar]
- 105.Nociari M., Ocheretina O., Schoggins J.W., Falck-Pedersen E. Sensing Infection by Adenovirus: Toll-Like Receptor-Independent Viral DNA Recognition Signals Activation of the Interferon Regulatory Factor 3 Master Regulator. J. Virol. 2007;81:4145–4157. doi: 10.1128/JVI.02685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zaiss A.K., Vilaysane A., Cotter M.J., Clark S.A., Meijndert H.C., Colarusso P., Yates R.M., Petrilli V., Tschopp J., Muruve D.A. Antiviral Antibodies Target Adenovirus to Phagolysosomes and Amplify the Innate Immune Response. J. Immunol. 2009;182:7058–7068. doi: 10.4049/jimmunol.0804269. [DOI] [PubMed] [Google Scholar]
- 107.Fejer G., Drechsel L., Liese J., Schleicher U., Ruzsics Z., Imelli N., Greber U.F., Keck S., Hildenbrand B., Krug A., et al. Key Role of Splenic Myeloid DCs in the IFN-αβ Response to Adenoviruses In Vivo. PLoS Pathog. 2008;4:e1000208. doi: 10.1371/journal.ppat.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tian J., Xu Z., Smith J.S., Hofherr S.E., Barry M.A., Byrnes A.P. Adenovirus Activates Complement by Distinctly Different Mechanisms In Vitro and In Vivo: Indirect Complement Activation by Virions In Vivo. J. Virol. 2009;83:5648–5658. doi: 10.1128/JVI.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arnberg N. Adenovirus receptors: Implications for tropism, treatment and targeting. Rev. Med. Virol. 2009;19:165–178. doi: 10.1002/rmv.612. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y., Chirmule N., Gao G.-P., Qian R., Croyle M., Joshi B., Tazelaar J., Wilson J.M. Acute Cytokine Response to Systemic Adenoviral Vectors in Mice Is Mediated by Dendritic Cells and Macrophages. Mol. Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 111.Muruve D.A., Barnes M.J., Stillman I.E., Libermann T.A. Adenoviral Gene Therapy Leads to Rapid Induction of Multiple Chemokines and Acute Neutrophil-Dependent Hepatic Injury In Vivo. Hum. Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 112.Schnell M.A., Zhang Y., Tazelaar J., Gao G.-P., Yu Q., Qian R., Chen S.-J., Varnavski A.N., LeClair C., Raper S.E., et al. Activation of Innate Immunity in Nonhuman Primates Following Intraportal Administration of Adenoviral Vectors. Mol. Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- 113.Zhu J., Huang X., Yang Y. A Critical Role for Type I IFN–dependent NK Cell Activation in Innate Immune Elimination of Adenoviral Vectors In Vivo. Mol. Ther. 2008;16:1300–1307. doi: 10.1038/mt.2008.88. [DOI] [PubMed] [Google Scholar]
- 114.Johnson M.J., Björkström N.K., Petrovas C., Liang F., Gall J.G., Loré K., Koup R.A. Type I interferon-dependent activation of NK cells by rAd28 or rAd35, but not rAd5, leads to loss of vector-insert expression. Vaccine. 2013;32:717–724. doi: 10.1016/j.vaccine.2013.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fausther-Bovendo H., Kobinger G.P. Pre-existing immunity against Ad vectors. Hum. Vaccines Immunother. 2014;10:2875–2884. doi: 10.4161/hv.29594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lowenstein P.R., Castro M.G. Inflammation and adaptive immune responses to adenoviral vectors injected into the brain: Peculiarities, mechanisms, and consequences. Gene Ther. 2003;10:946–954. doi: 10.1038/sj.gt.3302048. [DOI] [PubMed] [Google Scholar]
- 117.Zaiss A.K., Machado H.B., Herschman H.R. The influence of innate and pre-existing immunity on adenovirus therapy. J. Cell. Biochem. 2009;108:778–790. doi: 10.1002/jcb.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang Y., Li Q., Ertl H.C., Wilson J.M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sumida S.M., Truitt D.M., Kishko M.G., Arthur J.C., Jackson S.S., Gorgone D.A., Lifton M.A., Koudstaal W., Pau M.G., Kostense S., et al. Neutralizing Antibodies and CD8+ T Lymphocytes both Contribute to Immunity to Adenovirus Serotype 5 Vaccine Vectors. J. Virol. 2004;78:2666–2673. doi: 10.1128/JVI.78.6.2666-2673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rahman A., Tsai V., Goudreau A., Shinoda J., Wen S.F., Ramachandra M., Ralston R., Maneval D., LaFace D., Shabram P. Specific Depletion of Human Anti-adenovirus Antibodies Facilitates Transduction in an In Vivo Model for Systemic Gene Therapy. Mol. Ther. 2001;3:768–778. doi: 10.1006/mthe.2001.0316. [DOI] [PubMed] [Google Scholar]
- 121.Bradley R.R., Lynch D.M., Iampietro M.J., Borducchi E.N., Barouch D.H. Adenovirus Serotype 5 Neutralizing Antibodies Target both Hexon and Fiber following Vaccination and Natural Infection. J. Virol. 2012;86:625–629. doi: 10.1128/JVI.06254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sumida S.M., Truitt D.M., Lemckert A.A.C., Vogels R., Custers J.H.H.V., Addo M.M., Lockman S., Peter T., Peyerl F.W., Kishko M.G., et al. Neutralizing Antibodies to Adenovirus Serotype 5 Vaccine Vectors Are Directed Primarily against the Adenovirus Hexon Protein. J. Immunol. 2005;174:7179–7185. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- 123.Khare R., Reddy V.S., Nemerow G.R., Barry M.A. Identification of Adenovirus Serotype 5 Hexon Regions That Interact with Scavenger Receptors. J. Virol. 2012;86:2293–2301. doi: 10.1128/JVI.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bradley R.R., Maxfield L.F., Lynch D.M., Iampietro M.J., Borducchi E.N., Barouch D.H. Adenovirus Serotype 5-Specific Neutralizing Antibodies Target Multiple Hexon Hypervariable Regions. J. Virol. 2012;86:1267–1272. doi: 10.1128/JVI.06165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith J.G., Cassany A., Gerace L., Ralston R., Nemerow G.R. Neutralizing Antibody Blocks Adenovirus Infection by Arresting Microtubule-Dependent Cytoplasmic Transport. J. Virol. 2008;82:6492–6500. doi: 10.1128/JVI.00557-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gahéry-Ségard H., Farace F., Godfrin D., Gaston J., Lengagne R., Tursz T., Boulanger P., Guillet J.-G. Immune Response to Recombinant Capsid Proteins of Adenovirus in Humans: Antifiber and Anti-Penton Base Antibodies Have a Synergistic Effect on Neutralizing Activity. J. Virol. 1998;72:2388–2397. doi: 10.1128/JVI.72.3.2388-2397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tomita K., Sakurai F., Iizuka S., Hemmi M., Wakabayashi K., Machitani M., Tachibana M., Katayama K., Kamada H., Mizuguchi H. Antibodies against adenovirus fiber and penton base proteins inhibit adenovirus vector-mediated transduction in the liver following systemic administration. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-30947-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheng C., Gall J.G.D., Nason M., King C.R., Koup R.A., Roederer M., McElrath M.J., Morgan C.A., Churchyard G., Baden L.R., et al. Differential Specificity and Immunogenicity of Adenovirus Type 5 Neutralizing Antibodies Elicited by Natural Infection or Immunization. J. Virol. 2010;84:630–638. doi: 10.1128/JVI.00866-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sarkioja M., Pesonen S., Raki M., Hakkarainen T., Salo J., Ahonen M., Kanerva A., Hemminki A. Changing the adenovirus fiber for retaining gene delivery efficacy in the presence of neutralizing antibodies. Gene Ther. 2008;15:921–929. doi: 10.1038/gt.2008.56. [DOI] [PubMed] [Google Scholar]
- 130.Wohlfart C. Neutralization of adenoviruses: Kinetics, stoichiometry, and mechanisms. J. Virol. 1988;62:2321–2328. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boudin M.-L., Boulanger P. Antibody-triggered dissociation of adenovirus penton capsomer. Virology. 1981;113:781–786. doi: 10.1016/0042-6822(81)90208-7. [DOI] [PubMed] [Google Scholar]
- 132.Everitt E., De Luca A., Blixt Y. Antibody-mediated uncoating of adenovirus in vitro. FEMS Microbiol. Lett. 1992;98:21–27. doi: 10.1111/j.1574-6968.1992.tb05484.x. [DOI] [PubMed] [Google Scholar]
- 133.Varghese R., Mikyas Y., Stewart P.L., Ralston R. Postentry Neutralization of Adenovirus Type 5 by an Antihexon Antibody. J. Virol. 2004;78:12320–12332. doi: 10.1128/JVI.78.22.12320-12332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schirmbeck R., Reimann J., Kochanek S., Kreppel F. The Immunogenicity of Adenovirus Vectors Limits the Multispecificity of CD8 T-cell Responses to Vector-encoded Transgenic Antigens. Mol. Ther. 2008;16:1609–1616. doi: 10.1038/mt.2008.141. [DOI] [PubMed] [Google Scholar]
- 135.Flomenberg P., Piaskowski V., Truitt R.L., Casper J.T. Characterization of Human Proliferative T Cell Responses to Adenovirus. J. Infect. Dis. 1995;171:1090–1096. doi: 10.1093/infdis/171.5.1090. [DOI] [PubMed] [Google Scholar]
- 136.Olive M., Eisenlohr L.C., Flomenberg P. Quantitative Analysis of Adenovirus-Specific CD4+T-Cell Responses from Healthy Adults. Viral Immunol. 2001;14:403–413. doi: 10.1089/08828240152716646. [DOI] [PubMed] [Google Scholar]
- 137.Olive M., Eisenlohr L., Flomenberg N., Hsu S., Flomenberg P. The Adenovirus Capsid Protein Hexon Contains a Highly Conserved Human CD4+T-Cell Epitope. Hum. Gene Ther. 2002;13:1167–1178. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- 138.Tang J., Olive M., Champagne K., Flomenberg N., Eisenlohr L., Hsu S., Flomenberg P. Adenovirus hexon T-cell epitope is recognized by most adults and is restricted by HLA DP4, the most common class II allele. Gene Ther. 2004;11:1408–1415. doi: 10.1038/sj.gt.3302316. [DOI] [PubMed] [Google Scholar]
- 139.Leen A.M., Sili U., Vanin E.F., Jewell A.M., Xie W., Vignali D., Piedra P.A., Brenner M.K., Rooney C.M. Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood. 2004;104:2432–2440. doi: 10.1182/blood-2004-02-0646. [DOI] [PubMed] [Google Scholar]
- 140.Jooss K., Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: Implications for gene therapy. Gene Ther. 2003;10:955–963. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- 141.Kaech S.M., Tan J.T., Wherry E.J., Konieczny B.T., Surh C.D., Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 142.Arens R., Schoenberger S.P. Plasticity in programming of effector and memory CD8+T-cell formation. Immunol. Rev. 2010;235:190–205. doi: 10.1111/j.0105-2896.2010.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hutnick N.A., Carnathan D., Demers K., Makedonas G., Ertl H.C., Betts M.R. Adenovirus-specific human T cells are pervasive, polyfunctional, and cross-reactive. Vaccine. 2010;28:1932–1941. doi: 10.1016/j.vaccine.2009.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Loré K., Adams W.C., Havenga M.J.E., Precopio M.L., Holterman L., Goudsmit J., Koup R.A. Myeloid and Plasmacytoid Dendritic Cells Are Susceptible to Recombinant Adenovirus Vectors and Stimulate Polyfunctional Memory T Cell Responses. J. Immunol. 2007;179:1721–1729. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Penaloza-MacMaster P., Provine N.M., Ra J., Borducchi E.N., McNally A., Simmons N.L., Iampietro M.J., Barouch D.H. Alternative Serotype Adenovirus Vaccine Vectors Elicit Memory T Cells with Enhanced Anamnestic Capacity Compared to Ad5 Vectors. J. Virol. 2013;87:1373–1384. doi: 10.1128/JVI.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yun C.-O., Yoon A.-R., Yoo J.Y., Kim H., Kim M., Ha T., Kim G.E., Kim H., Kim J.-H. Coxsackie and Adenovirus Receptor Binding Ablation Reduces Adenovirus Liver Tropism and Toxicity. Hum. Gene Ther. 2005;16:248–261. doi: 10.1089/hum.2005.16.248. [DOI] [PubMed] [Google Scholar]
- 147.Wood M., Perrotte P., Onishi E., E Harper M., Dinney C., Pagliaro L., Wilson D.R. Biodistribution of an adenoviral vector carrying the luciferase reporter gene following intravesical or intravenous administration to a mouse. Cancer Gene Ther. 1999;6:367–372. doi: 10.1038/sj.cgt.7700090. [DOI] [PubMed] [Google Scholar]
- 148.Vigne E., Dedieu J.-F., Brie A., Gillardeaux A., Briot D., Benihoud K., Latta-Mahieu M., Saulnier P., Perricaudet M., Yeh P. Genetic manipulations of adenovirus type 5 fiber resulting in liver tropism attenuation. Gene Ther. 2003;10:153–162. doi: 10.1038/sj.gt.3301845. [DOI] [PubMed] [Google Scholar]
- 149.Zhang Z., Krimmel J., Zhang Z., Hu Z., Seth P. Systemic Delivery of a Novel Liver-Detargeted Oncolytic Adenovirus Causes Reduced Liver Toxicity but Maintains the Antitumor Response in a Breast Cancer Bone Metastasis Model. Hum. Gene Ther. 2011;22:1137–1142. doi: 10.1089/hum.2011.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alemany R., Curiel D. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 2001;8:1347–1353. doi: 10.1038/sj.gt.3301515. [DOI] [PubMed] [Google Scholar]
- 151.Shayakhmetov D.M., Gaggar A., Ni S., Li Z.-Y., Lieber A. Adenovirus Binding to Blood Factors Results in Liver Cell Infection and Hepatotoxicity. J. Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Neels J.G., van Den Berg B.M., Mertens K., ter Maat H., Pannekoek H., van Zonneveld A.J., Lenting P.J. Activation of factor IX zymogen results in exposure of a binding site for low-density lipoprotein receptor-related protein. Blood. 2000;96:3459–3465. doi: 10.1182/blood.V96.10.3459. [DOI] [PubMed] [Google Scholar]
- 153.Parker A.L., Waddington S.N., Nicol C.G., Shayakhmetov D.M., Buckley S.M., Denby L., Kemball-Cook G., Ni S., Lieber A., McVey J.H., et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- 154.Lieber A., He C.Y., Meuse L., Schowalter D., Kirillova I., Winther B., Kay M.A. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Allen R.J., Byrnes A.P. Interaction of adenovirus with antibodies, complement, and coagulation factors. FEBS Lett. 2019;593:3449–3460. doi: 10.1002/1873-3468.13649. [DOI] [PubMed] [Google Scholar]
- 156.Waddington S.N., McVey J.H., Bhella D., Parker A.L., Barker K., Atoda H., Pink R., Buckley S.M.K., Greig J.A., Denby L., et al. Adenovirus Serotype 5 Hexon Mediates Liver Gene Transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 157.Alba R., Bradshaw A.C., Parker A.L., Bhella D., Waddington S.N., Nicklin S.A., van Rooijen N., Custers J., Goudsmit J., Barouch D.H., et al. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: Effect of mutagenesis on FX interactions and gene transfer. Blood. 2009;114:965–971. doi: 10.1182/blood-2009-03-208835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lenman A., Müller S., Nygren M.I., Frängsmyr L., Stehle T., Arnberg N. Coagulation Factor IX Mediates Serotype-Specific Binding of Species A Adenoviruses to Host Cells. J. Virol. 2011;85:13420–13431. doi: 10.1128/JVI.06088-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Koizumi N., Yamaguchi T., Kawabata K., Sakurai F., Sasaki T., Watanabe Y., Hayakawa T., Mizuguchi H. Fiber-Modified Adenovirus Vectors Decrease Liver Toxicity through Reduced IL-6 Production. J. Immunol. 2007;178:1767–1773. doi: 10.4049/jimmunol.178.3.1767. [DOI] [PubMed] [Google Scholar]
- 160.Tessarollo N., Domingues A., Antunes F., Luz J., Rodrigues O., Cerqueira O., Strauss B. Nonreplicating Adenoviral Vectors: Improving Tropism and Delivery of Cancer Gene Therapy. Cancers. 2021;13:1863. doi: 10.3390/cancers13081863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yamamoto Y., Nagasato M., Yoshida T., Aoki K. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci. 2017;108:831–837. doi: 10.1111/cas.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Beatty M.S., Curiel D.T. Adenovirus Strategies for Tissue-Specific Targeting. Adv. Cancer Res. 2012;115:39–67. doi: 10.1016/b978-0-12-398342-8.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mennechet F.J.D., Paris O., Ouoba A.R., Arenas S.S., Sirima S.B., Dzomo G.R.T., Diarra A., Traore I.T., Kania D., Eichholz K., et al. A review of 65 years of human adenovirus seroprevalence. Expert Rev. Vaccines. 2019;18:597–613. doi: 10.1080/14760584.2019.1588113. [DOI] [PubMed] [Google Scholar]
- 164.Thorner A.R., Vogels R., Kaspers J., Weverling G.J., Holterman L., Lemckert A.A.C., Dilraj A., McNally L.M., Jeena P.M., Jepsen S., et al. Age Dependence of Adenovirus-Specific Neutralizing Antibody Titers in Individuals from Sub-Saharan Africa. J. Clin. Microbiol. 2006;44:3781–3783. doi: 10.1128/JCM.01249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ledgerwood J.E., Costner P., Desai N., Holman L., Enama M.E., Yamshchikov G., Mulangu S., Hu Z., Andrews C.A., Sheets R.A., et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29:304–313. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 166.Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., Jiang T., Hou L.-H., Li J.-X., Yang B.-F., Wang L., Wang W.-J., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pandey A., Singh N., Vemula S.V., Couëtil L., Katz J.M., Donis R., Sambhara S., Mittal S.K. Impact of Preexisting Adenovirus Vector Immunity on Immunogenicity and Protection Conferred with an Adenovirus-Based H5N1 Influenza Vaccine. PLoS ONE. 2012;7:e33428. doi: 10.1371/journal.pone.0033428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang Q., Seto D. Chimpanzee Adenovirus Vector Ebola Vaccine—Preliminary Report. N. Engl. J. Med. 2015;373:775–776. doi: 10.1056/nejmc1505499. [DOI] [PubMed] [Google Scholar]
- 169.Sayedahmed E.E., Kumari R., Shukla S., Hassan A.O., Mohammed S.I., York I.A., Gangappa S., Sambhara S., Mittal S.K. Longevity of adenovirus vector immunity in mice and its implications for vaccine efficacy. Vaccine. 2018;36:6744–6751. doi: 10.1016/j.vaccine.2018.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Barouch D.H., Pau M.G., Custers J.H.H.V., Koudstaal W., Kostense S., Havenga M.J.E., Truitt D.M., Sumida S.M., Kishko M.G., Arthur J.C., et al. Immunogenicity of Recombinant Adenovirus Serotype 35 Vaccine in the Presence of Pre-Existing Anti-Ad5 Immunity. J. Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 171.Jones I., Roy P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet. 2021;397:671. doi: 10.1016/S0140-6736(21)00191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Reddy P.S., Ganesh S., Limbach M., Brann T., Pinkstaff A., Kaloss M., Kaleko M., Connelly S. Development of adenovirus serotype 35 as a gene transfer vector. Virology. 2003;311:384–393. doi: 10.1016/S0042-6822(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 173.Stone D., Ni S., Li Z.-Y., Gaggar A., DiPaolo N., Feng Q., Sandig V., Lieber A. Development and Assessment of Human Adenovirus Type 11 as a Gene Transfer Vector. J. Virol. 2005;79:5090–5104. doi: 10.1128/JVI.79.8.5090-5104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Abbink P., Lemckert A.A.C., Ewald B.A., Lynch D.M., Denholtz M., Smits S., Holterman L., Damen I., Vogels R., Thorner A.R., et al. Comparative Seroprevalence and Immunogenicity of Six Rare Serotype Recombinant Adenovirus Vaccine Vectors from Subgroups B and D. J. Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tameris M., Hokey D., Nduba V., Sacarlal J., Laher F., Kiringa G., Gondo K., Lazarus E., Gray G., Nachman S., et al. A double-blind, randomised, placebo-controlled, dose-finding trial of the novel tuberculosis vaccine AERAS-402, an adenovirus-vectored fusion protein, in healthy, BCG-vaccinated infants. Vaccine. 2015;33:2944–2954. doi: 10.1016/j.vaccine.2015.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ouédraogo A., Tiono A.B., Kargougou D., Yaro J.B., Ouédraogo E., Kaboré Y., Kangoye D., Bougouma E.C., Gansane A., Henri N., et al. A Phase 1b Randomized, Controlled, Double-Blinded Dosage-Escalation Trial to Evaluate the Safety, Reactogenicity and Immunogenicity of an Adenovirus Type 35 Based Circumsporozoite Malaria Vaccine in Burkinabe Healthy Adults 18 to 45 Years of Age. PLoS ONE. 2013;8:e78679. doi: 10.1371/journal.pone.0078679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Keefer M.C., Gilmour J., Hayes P., Gill D., Kopycinski J., Cheeseman H., Cashin-Cox M., Naarding M., Clark L., Fernández N., et al. A Phase I Double Blind, Placebo-Controlled, Randomized Study of a Multigenic HIV-1 Adenovirus Subtype 35 Vector Vaccine in Healthy Uninfected Adults. PLoS ONE. 2012;7:e41936. doi: 10.1371/journal.pone.0041936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Baden L.R., Walsh S.R., Seaman M.S., Tucker R.P., Krause K.H., Patel A., Johnson J.A., Kleinjan J., Yanosick K.E., Perry J., et al. First-in-Human Evaluation of the Safety and Immunogenicity of a Recombinant Adenovirus Serotype 26 HIV-1 Env Vaccine (IPCAVD 001) J. Infect. Dis. 2012;207:240–247. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Matz K.M., Marzi A., Feldmann H. Ebola vaccine trials: Progress in vaccine safety and immunogenicity. Expert Rev. Vaccines. 2019;18:1229–1242. doi: 10.1080/14760584.2019.1698952. [DOI] [PubMed] [Google Scholar]
- 180.Mendonça S.A., Lorincz R., Boucher P., Curiel D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. Npj Vaccines. 2021;6:1–14. doi: 10.1038/s41541-021-00356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brouwer E., Havenga M.J., Ophorst O., De Leeuw B., Gijsbers L., Gillissen G., Hoeben R., Ter Horst M., Nanda D., Dirven C., et al. Human adenovirus type 35 vector for gene therapy of brain cancer: Improved transduction and bypass of pre-existing anti-vector immunity in cancer patients. Cancer Gene Ther. 2006;14:211–219. doi: 10.1038/sj.cgt.7701010. [DOI] [PubMed] [Google Scholar]
- 182.Barouch D.H., Liu J., Peter L., Abbink P., Iampietro M.J., Cheung A., Alter G., Chung A., Dugast A.-S., Frahm N., et al. Characterization of Humoral and Cellular Immune Responses Elicited by a Recombinant Adenovirus Serotype 26 HIV-1 Env Vaccine in Healthy Adults (IPCAVD 001) J. Infect. Dis. 2012;207:248–256. doi: 10.1093/infdis/jis671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Teigler J.E., Iampietro M.J., Barouch D.H. Vaccination with Adenovirus Serotypes 35, 26, and 48 Elicits Higher Levels of Innate Cytokine Responses than Adenovirus Serotype 5 in Rhesus Monkeys. J. Virol. 2012;86:9590–9598. doi: 10.1128/JVI.00740-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ahi Y.S., Bangari D.S., Mittal S.K. Adenoviral Vector Immunity: Its Implications and Circumvention Strategies. Curr. Gene Ther. 2011;11:307–320. doi: 10.2174/156652311796150372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xiang Z., Li Y., Cun A., Yang W., Ellenberg S., Switzer W.M., Kalish M.L., Ertl H.C. Chimpanzee Adenovirus Antibodies in Humans, Sub-Saharan Africa. Emerg. Infect. Dis. 2006;12:1596–1599. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Colloca S., Barnes E., Folgori A., Ammendola V., Capone S., Cirillo A., Siani L., Naddeo M., Grazioli F., Esposito M.L., et al. Vaccine Vectors Derived from a Large Collection of Simian Adenoviruses Induce Potent Cellular Immunity Across Multiple Species. Sci. Transl. Med. 2012;4:115ra2. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Alhashimi M., Elkashif A., Sayedahmed E., Mittal S. Nonhuman Adenoviral Vector-Based Platforms and Their Utility in Designing Next Generation of Vaccines for Infectious Diseases. Viruses. 2021;13:1493. doi: 10.3390/v13081493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fitzgerald J., Gao G.-P., Reyes-Sandoval A., Pavlakis G.N., Xiang Z.Q., Wlazlo A.P., Giles-Davis W., Wilson J.M., Ertl H.C.J. A Simian Replication-Defective Adenoviral Recombinant Vaccine to HIV-1 Gag. J. Immunol. 2003;170:1416–1422. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- 189.Guo J., Mondal M., Zhou D. Development of novel vaccine vectors: Chimpanzee adenoviral vectors. Hum. Vaccines Immunother. 2018;14:1679–1685. doi: 10.1080/21645515.2017.1419108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tapia M.D., Sow O.S., E Lyke K., Haidara F.C., Diallo F., Doumbia M., Traore A., Coulibaly F., Kodio M., Onwuchekwa U., et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: A phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2015;16:31–42. doi: 10.1016/s1473-3099(15)00362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rampling T., Ewer K.J., Bowyer G., Bliss C.M., Edwards N.J., Wright D., Payne R.O., Venkatraman N., de Barra E., Snudden C.M., et al. Safety and High Level Efficacy of the Combination Malaria Vaccine Regimen of RTS,S/AS01B With Chimpanzee Adenovirus 63 and Modified Vaccinia Ankara Vectored Vaccines Expressing ME-TRAP. J. Infect. Dis. 2016;214:772–781. doi: 10.1093/infdis/jiw244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hayton E.-J., Rose A., Ibrahimsa U., Del Sorbo M., Capone S., Crook A., Black A.P., Dorrell L., Hanke T. Safety and Tolerability of Conserved Region Vaccines Vectored by Plasmid DNA, Simian Adenovirus and Modified Vaccinia Virus Ankara Administered to Human Immunodeficiency Virus Type 1-Uninfected Adults in a Randomized, Single-Blind Phase I Trial. PLoS ONE. 2014;9:e101591. doi: 10.1371/journal.pone.0101591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Green A.C., Scarselli E., Voysey M., Capone S., Vitelli A., Nicosia A., Cortese R., Thompson A.J., Sande C., De Lara C., et al. Safety and immunogenicity of novel respiratory syncytial virus (RSV) vaccines based on the RSV viral proteins F, N and M2-1 encoded by simian adenovirus (PanAd3-RSV) and MVA (MVA-RSV); protocol for an open-label, dose-escalation, single-centre, phase 1 clinical trial in healthy adults. BMJ Open. 2015;5:e008748. doi: 10.1136/bmjopen-2015-008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reddy P.S., Idamakanti N., Chen Y., Whale T., Babiuk L.A., Mehtali M., Tikoo S.K. Replication-Defective Bovine Adenovirus Type 3 as an Expression Vector. J. Virol. 1999;73:9137–9144. doi: 10.1128/JVI.73.11.9137-9144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tandon M., Sharma A., Vemula S.V., Bangari D.S., Mittal S.K. Sequential administration of bovine and human adenovirus vectors to overcome vector immunity in an immunocompetent mouse model of breast cancer. Virus Res. 2012;163:202–211. doi: 10.1016/j.virusres.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sharma A., Tandon M.S., Ahi Y., Bangari D.S., Vemulapalli R., Mittal S.K. Evaluation of cross-reactive cell-mediated immune responses among human, bovine and porcine adenoviruses. Gene Ther. 2010;17:634–642. doi: 10.1038/gt.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sharma A., Bangari D.S., Tandon M., Pandey A., HogenEsch H., Mittal S.K. Comparative analysis of vector biodistribution, persistence and gene expression following intravenous delivery of bovine, porcine and human adenoviral vectors in a mouse model. Virology. 2009;386:44–54. doi: 10.1016/j.virol.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sharma A., Bangari D.S., Tandon M., HogenEsch H., Mittal S.K. Evaluation of innate immunity and vector toxicity following inoculation of bovine, porcine or human adenoviral vectors in a mouse model. Virus Res. 2010;153:134–142. doi: 10.1016/j.virusres.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sayedahmed E.E., Hassan A.O., Kumari R., Cao W., Gangappa S., York I., Sambhara S., Mittal S.K. A Bovine Adenoviral Vector-Based H5N1 Influenza -Vaccine Provides Enhanced Immunogenicity and Protection at a Significantly Low Dose. Mol. Ther.—Methods Clin. Dev. 2018;10:210–222. doi: 10.1016/j.omtm.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Khan A., Sayedahmed E.E., Singh V.K., Mishra A., Dorta-Estremera S., Nookala S., Canaday D.H., Chen M., Wang J., Sastry K.J., et al. A recombinant bovine adenoviral mucosal vaccine expressing mycobacterial antigen-85B generates robust protection against tuberculosis in mice. Cell Rep. Med. 2021;2:100372. doi: 10.1016/j.xcrm.2021.100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Roberts D.M., Nanda A., Havenga M.J.E., Abbink P., Lynch D.M., Ewald B.A., Liu J., Thorner A.R., Swanson P.E., Gorgone D.A., et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 202.Flickinger J.C., Jr., Singh J., Carlson R., Leong E., Baybutt T.R., Barton J., Caparosa E., Pattison A., A Rappaport J., Roh J., et al. Chimeric Ad5.F35 vector evades anti-adenovirus serotype 5 neutralization opposing GUCY2C-targeted antitumor immunity. J. Immunother. Cancer. 2020;8:e001046. doi: 10.1136/jitc-2020-001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rogée S., E Grellier E., Bernard C., Jouy N., A Loyens A., Beauvillain J.C., Fender P., Corjon S., Hong S.S., Boulanger P., et al. Influence of chimeric human-bovine fibers on adenoviral uptake by liver cells and the antiviral immune response. Gene Ther. 2010;17:880–891. doi: 10.1038/gt.2010.37. [DOI] [PubMed] [Google Scholar]
- 204.Gao J., Zhang W., Mese K., Bunz O., Lu F., Ehrhardt A. Transient Chimeric Ad5/37 Fiber Enhances NK-92 Carrier Cell-Mediated Delivery of Oncolytic Adenovirus Type 5 to Tumor Cells. Mol. Ther.—Methods Clin. Dev. 2020;18:376–389. doi: 10.1016/j.omtm.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Stepanenko A.A., Chekhonin V.P. Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX. Virus Res. 2018;257:40–51. doi: 10.1016/j.virusres.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 206.O’Riordan C.R., Lachapelle A., Delgado C., Parkes V., Wadsworth S.C., Smith A.E., Francis G. PEGylation of Adenovirus with Retention of Infectivity and Protection from Neutralizing Antibody In Vitro and In Vivo. Hum. Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- 207.A Croyle M., Le H.T., Linse K.D., Cerullo V., Toietta G., Beaudet A., Pastore L. PEGylated helper-dependent adenoviral vectors: Highly efficient vectors with an enhanced safety profile. Gene Ther. 2005;12:579–587. doi: 10.1038/sj.gt.3302441. [DOI] [PubMed] [Google Scholar]
- 208.Mok H., Park J.W., Park T.G. Microencapsulation of PEGylated Adenovirus within PLGA Microspheres for Enhanced Stability and Gene Transfection Efficiency. Pharm. Res. 2007;24:2263–2269. doi: 10.1007/s11095-007-9441-y. [DOI] [PubMed] [Google Scholar]
- 209.Yotnda P., Chen D.-H., Chiu W., A Piedra P., Davis A., Templeton N.S., Brenner M.K. Bilamellar Cationic Liposomes Protect Adenovectors from Preexisting Humoral Immune Responses. Mol. Ther. 2002;5:233–241. doi: 10.1006/mthe.2002.0545. [DOI] [PubMed] [Google Scholar]
- 210.Fukuhara H., Hayashi Y., Yamamoto N., Fukui T., Nishikawa M., Mitsudo K., Tohnai I., Ueda M., Mizuno M., Yoshida J. Improvement of transduction efficiency of recombinant adenovirus vector conjugated with cationic liposome for human oral squamous cell carcinoma cell lines. Oral Oncol. 2003;39:601–609. doi: 10.1016/S1368-8375(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 211.Zhong Z., Shi S., Han J., Zhang Z., Sun X. Anionic Liposomes Increase the Efficiency of Adenovirus-Mediated Gene Transfer to Coxsackie-Adenovirus Receptor Deficient Cells. Mol. Pharm. 2010;7:105–115. doi: 10.1021/mp900151k. [DOI] [PubMed] [Google Scholar]
- 212.Han S.-Y., Lee Y.-J., Jung H.-I., Lee S.-W., Lim S.-J., Hong S.-H., Jeong J.-S. Gene transfer using liposome-complexed adenovirus seems to overcome limitations due to coxsackievirus and adenovirus receptor-deficiency of cancer cells, both in vitro and in vivo. Exp. Mol. Med. 2008;40:427–434. doi: 10.3858/emm.2008.40.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vupputuri S., Tayebi L., Koralege R.S.H., Nigatu A., Mozafari M., Mishra A., Liu L., Ramsey J.D. Polyethylene glycol–modified DOTAP:cholesterol/adenovirus hybrid vectors have improved transduction efficiency and reduced immunogenicity. J. Nanoparticle Res. 2021;23:1–14. doi: 10.1007/s11051-020-05134-9. [DOI] [Google Scholar]
- 214.Croyle M.A., Patel A., Tran K.N., Gray M., Zhang Y., Strong J.E., Feldmann H., Kobinger G.P. Nasal Delivery of an Adenovirus-Based Vaccine Bypasses Pre-Existing Immunity to the Vaccine Carrier and Improves the Immune Response in Mice. PLoS ONE. 2008;3:e3548. doi: 10.1371/journal.pone.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xiang Z.Q., Gao G.P., Reyes-Sandoval A., Li Y., Wilson J.M., Ertl H.C.J. Oral Vaccination of Mice with Adenoviral Vectors Is Not Impaired by Preexisting Immunity to the Vaccine Carrier. J. Virol. 2003;77:10780–10789. doi: 10.1128/JVI.77.20.10780-10789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.