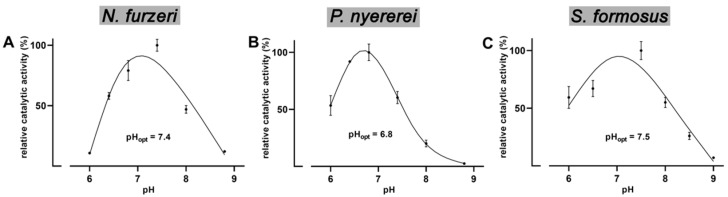
Figure 6.
pH-dependence of AA oxygenation catalyzed by the different bony fish ALOX isoforms. The lysate supernatants were used as enzyme source and activity assays were carried out at different pH values. As reaction buffers equimolar mixtures of 10 mM phosphate buffer and 10 mM borate buffer were used and different pH-values were adjusted with 5 N HCl or 5 N NaOH at room temperature. The amounts of ALOX products formed during a 3 min incubation period were quantified by RP-HPLC Two independent activity assays were carried out at room temperature at each pH and means and error bars are indicated. The catalytic activity at pHopt for each enzyme was set 100%. (A) N. furzeri, (B) P. nyererei, (C) S. formosus. Correlation coefficients R2 > 0.9 (R2 for N. furzeri = 0.971, R2 for P. nyererei = 0.986, R2 for S. formosus = 0.951).