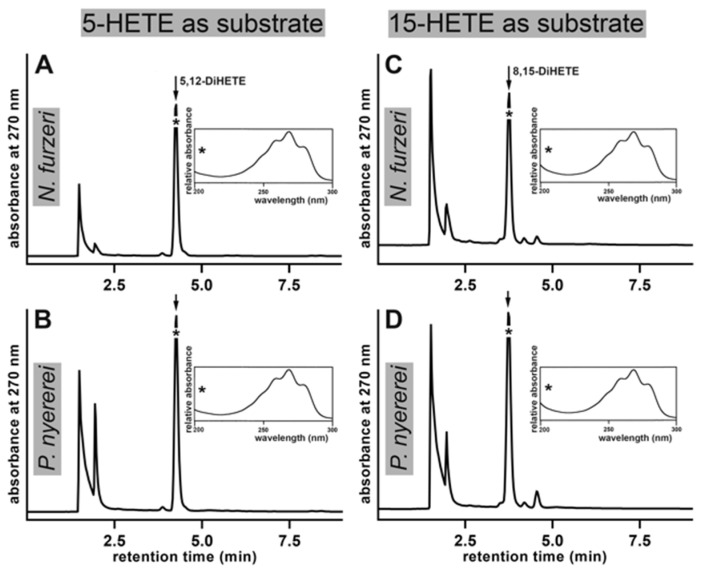
Figure 8.
Oxygenation of 5R/S-HETE, 15R/S-HETE by bony fish ALOX-isoforms. ALOX activity assays (substrate concentration 30 µM) were carried out using the cellular lysate supernatants as enzyme source. After 10 min of incubation the reaction products were reduced and analyzed by RP-HPLC recording the absorbance at 270 nm. The uv-spectra of the major oxygenation products were taken during the chromatographic run at the time points indicated (*). The chemical structures of the major oxygenation products were confirmed by LC-MS analysis. (A) N. furzeri ALOX, 5-HETE as substrate, (B) P. nyererei ALOX, 5-HETE as substrate, (C) N. furzeri ALOX, 15-HETE, as substrate (D) P. nyererei ALOX, 15-HETE as substrate. For better comparison of the product profiles each chromatogram was scaled to the highest conjugated triene peak. Thus, the amounts of products formed by the two enzymes cannot directly be compared.