Abstract
Mammalian communities inhabiting temperate grasslands are of conservation concern globally, especially in Central Asia, where livestock numbers have dramatically increased in recent decades, leading to overgrazing and land‐use change. Yet, how this pervasive presence of livestock herds affects the community of wild mammals remains largely unstudied. We used systematic camera trapping at 216 sites across remote, mountainous areas of the Mongolian Altai Mountains to assess the spatial and temporal patterns of occurrence and the interspecific relationships within a mammalian community that includes different categories of livestock. By adopting a recently proposed multispecies occupancy model that incorporates interspecific correlation in occupancy, we found several statistically strong correlations in occupancy among species pairs, with the majority involving livestock. The sign of such associations was markedly species‐dependent, with larger wild species of conservation concern, namely, snow leopard and Siberian ibex, avoiding livestock presence. As predicted, we found evidence of a positive correlation in occupancy between predators and their respective main prey. Contrary to our expectations, a number of intraguild species pairs also showed positive co‐occurrence, with no evidence of spatiotemporal niche partitioning. Overall, our study suggests that livestock encroaching into protected areas influences the whole local community of wild mammals. Though pastoralism has coexisted with wildlife for millennia in central Asian grasslands, our findings suggest that policies and practices to decrease the pressure of livestock husbandry on wildlife are needed, with special attention on large species, such as the snow leopard and its wild prey, which seem to be particularly sensitive to this pervasive livestock presence.
Keywords: activity pattern, community occupancy, grassland, interspecific interactions, livestock encroachment, snow leopard
INTRODUCTION
Species are not distributed randomly across space; rather, they form communities whose structure and composition are molded by biotic interactions (Davis et al., 2018; Wisz et al., 2013). With the dramatic increase of the human footprint on the planet (Venter et al., 2016), however, anthropogenic disturbance, particularly land‐use change, has become a prominent factor altering interspecific interactions and even community composition (e.g., Kiffner et al., 2015; Rovero et al., 2020; Suraci et al., 2021). Human activities such as hunting can, for example, affect mammalian communities through apex predator removal, causing the increase of large herbivore density and, in turn, a change in vegetation (Hebblewhite et al., 2005). Top‐down perturbations can also lead to mesopredator release with greater pressure on small prey and other alterations of ecosystem functioning (Brook et al., 2012; Newsome et al., 2017). Another relevant anthropogenic activity is livestock grazing; in some regions, like central Asia, it has increased steadily in recent decades, raising concerns over the effects it may have on communities of wild mammals (e.g., Berger et al., 2013). However, how grazing livestock affects the spatiotemporal occurrence and interspecific interactions of whole communities of mammals remains largely unstudied.
Mammalian communities inhabiting temperate grasslands are indeed of particular conservation concern globally because they constitute one of the most endangered and understudied biomes worldwide, threatened by land‐use change, overgrazing by livestock, and climate change (Hoekstra et al., 2005; Nunez et al., 2020; Richter & Osborne, 2014). Palearctic prairies are characterized by strong seasonal variations in precipitation and temperature—and, hence, in grass greenness and palatability—driving herbivores' use of space (Mueller et al., 2008). On the other hand, pastures are affected by herbivore grazing; for example, in Central Asia pikas (Ochotona spp.) and Brandt's voles (Lasiopodomys brandtii) impact vegetation community structure and composition through burrowing (Bagchi et al., 2006; Sawamukai et al., 2012). The presence and density of herbivores in turn influence the distribution and abundance of carnivores (Chetri et al., 2017; Ross et al., 2010a). In these grasslands, livestock grazing is a major factor at play, especially in Central Asia, where the number of domestic animals has dramatically increased in recent decades following globalization in the cashmere wool market (Berger et al., 2013). The detrimental effects of livestock encroachment and competition on mammals have been documented, particularly on wild ungulates (Ekernas et al., 2017; Mishra et al., 2004) and large carnivores (Sharma et al., 2015). However, the overall effect on the whole community of medium‐size and large mammals is poorly understood, and evidence shows that livestock may also alter interactions among wild species (e.g., Feng et al., 2021; Rottstock et al., 2020).
In this study, we aimed to assess the spatial and temporal patterns of occurrence and the interspecific relationships within a mammalian community in Central Asia that includes livestock. We sampled four areas in remote and rugged environments within the Mongolian Altai Mountains through systematic camera trapping at a total of 216 sampling sites. We took advantage of the ability of camera traps to detect multiple species, including domestic ones, simultaneously and at the same spatial scale. We analyzed data using a recently proposed multispecies occupancy model that accounts for imperfect detection and incorporates interspecific correlations in occupancy (Tobler et al., 2019). This analytical approach allowed us to study the effects of both environmental and anthropogenic factors on occupancy at both the community and species levels while quantifying the strength of potential interactions among species, as inferred from patterns of species co‐occurrence (Richmond et al., 2010).
Our main research questions and predictions were as follows: (1) Is there strong evidence for spatiotemporal interactions between wildlife and livestock? We predicted a generally negative co‐occurrence of wild species with livestock. (2) If associations between livestock and wild species exist, are they related to species' body size? We predicted that larger species would display more negative associations with livestock. (3) Do predators and prey in the community co‐occur in space and time? We expected a positive co‐occurrence of predators and their respective prey. (4) Is there evidence of intraguild competition through spatial or temporal avoidance? We predicted a negative co‐occurrence in space or time of species of the same guild as a result of niche partitioning.
METHODS
Study areas
This study was conducted in the Mongolian Altai Mountains in the western provinces of Bayan Olgii and Hovd, which are characterized by a mosaic of high plateaus and mountain ranges. The climate is semiarid, with short summers and long, cold, and dry winters. The dominant vegetation cover is steppe grassland, with sparse conifers and shrubs along valley bottoms and lower‐elevation slopes. We surveyed one area per year during spring (March–June) from 2015 to 2019, except 2016. Study areas (Figure 1) included (1) Siilkhem‐B National Park (49°49′ N; 89°44′ E) in northwestern Mongolia bordering Russia, with the highest elevation up to 3900 m above sea level (a.s.l.). This protected area (PA) includes grassland habitat along rocky slopes and sparse larches Larix sibirica along valley floors; (2) Tavan Bogd National Park (48°33′ N; 88°37′ E), in western Mongolia bordering Russia and China; this is the largest PA in Mongolia and hosts the highest mountain in the country (4374 m a.s.l.). The portion that we surveyed is characterized by alpine and glacial habitat at the highest elevations and grasslands at lower altitudes; (3) Khork Serkhe Strictly Protected Area (47°93′ N; 90°99′ E), located in Western Mongolia and reaching 4127 m a.s.l. Its mountainous landscape is shaped by an alternation of slopes and valleys; (4) Sutai massif (46°37′ N; 93°35′ E), which had no legal protection when surveyed in 2019 but was declared a Natural Reserve in 2020. Sutai reaches 4220 m a.s.l. at the highest peak, covered by the southernmost glacier of the region. More details on sampling areas and efforts are provided in Appendix S1.
FIGURE 1.
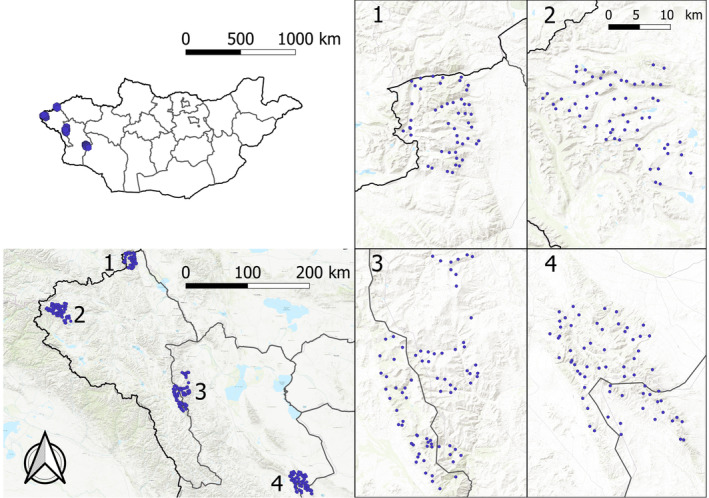
Map of the four study areas in the Altai Mountains of western Mongolia where wild and domestic mammals were detected by camera traps at 216 sites (blue dots): (1) Siilkhem‐B National Park, (2) Tavan Bogd National Park, (3) Khork Serkhe Strictly Protected Area, and (4) Sutai massif. Black lines represent national (thick) and provincial (thin) borders.
In the four study areas most inhabitants are nomadic herders. The pastoralists in the Sutai massif belong to the ethnic group of the Mongols and profess Buddhism, but in other areas they mainly belong to the ethnic group of the Kazakhs, professing Islam. In the sole Bayan Olgii province, where the first three surveyed areas occur, 2.2 million livestock were reported in 2018, 1.9 million of which were sheep and goats (National Statistics Office of Mongolia, https://www.en.nso.mn/). Large livestock, represented by horses, camels, cattle, and yaks, are mostly free ranging, whereas sheep and goats are guarded by herders and dogs during the day and held in corrals at night, except in summer (July–August), where usually no fence is used (Augugliaro et al., 2020). Mongolian National Parks are subdivided into three zones with different regulations: special zone, travel/tourism zone, and limited‐use zone. Traditional pastoralism is only allowed in the limited‐use zone, whereas it is not allowed at all in the Strictly Protected Areas.
Data collection: Camera trapping and covariates
For each area we first created in a geographic information system (GIS) a regular grid with cells of 4 km2 that aimed to cover at least the minimum extent of 500 km2 recommended for snow leopard (Panthera uncia) population estimate studies (Jackson et al., 2005). Within each cell we located in the field one camera‐trap site. The number and locations of chosen sites were constrained by terrain morphology, snow depth, and the number of camera traps available for sampling (Appendix S1: Table S1). Two of the PAs were too large to monitor in their entirety, so we selected a portion that we considered optimal habitat for the snow leopard according to previous research and local knowledge. Specifically, in Siilkhem‐B we did not sample the southern end of the PA, which includes low elevation and less suitable habitat, and in Tavan Bogd we focused on the northeastern part of the PA since the southern part hosts mainly coniferous forests. In Khork Serkhe we sampled the whole PA and surrounding suitable areas, and in Sutai we sampled the entire mountain massif. Overall, the area sampled ranged in size from 513 to 1110 km2 (Appendix S1: Table S1). As a result, we sampled with camera traps a total of 216 sites (48, 44, 63, and 61 for the four areas, respectively) that ran on average for 65 days each (SD = 20.7). The minimum distance between contiguous sites was 1.5 km. We placed camera traps on small rock piles around 50 cm above the ground at a distance of approximately 2–4 m from a target trail, setting no delay between consecutive triggers. Sites were usually located on passes, ridges, narrow passages, or valley bottoms and were chosen to maximize the detection of snow leopards, which we considered to be the most elusive species. However, our data show that these sites were used by several medium‐size to large mammals and by livestock. We did not use any baits or lures. Camera‐trap models were of three different manufacturers, characterized by high (Reconyx) and medium (Cuddeback and Bushnell) trigger speed. We annotated camera‐trap images using the software Wild.ID (Fegraus & MacCharty, 2016) and screened the data to derive for each area a checklist of species and their total number of detections. We set the temporal resolution of detections to 1 day, so repeated detections of the same species on the same day were counted as a single detection event, as is common practice in occupancy modeling.
Using a digital elevation model and a satellite map in the software Quantum GIS (QGIS, 2018) we derived three environmental covariates for each camera‐trap site: elevation, terrain slope, and distance from closest herder settlement (consisting of gers, houses, villages, or mining camps), whose geographic localization was acquired in the field with a global positioning satellite (GPS) device.
Data analyses
Out of all species detected (Appendix S1: Table S2) we excluded those found in only 1 or 2 areas, which generally yielded <5 detections (namely, Ursus arctos, Lynx lynx, Ovis ammon, Mustela erminea, and Sciurus vulgaris), and focused on the following 12 species of medium‐size to large mammals detected across at least 3 areas: 1 large herbivore (Capra sibirica), 3 large carnivores (Canis lupus, Panthera uncia, Gulo gulo), 4 medium‐size carnivores (Martes foina, Mustela eversmanni, Otocolobus manul, Vulpes vulpes), and 4 medium‐size to small herbivores (Lepus spp., Marmota spp., Ochotona spp., Spermophilus spp.). The last four were either represented by more than one species of the same genus occurring in different areas (i.e., Marmota sibirica was found in all areas except for Tavan Bogd NP, where M. baibacina occurs, and Lepus timidus was found in the first two areas, whereas L. tolai was found in the latter two) or occurring in the same area but indistinguishable from one another in camera‐trap photographs. Hence, given the similar ecology of these congeneric species, we merged them at the genus level. Moreover, we merged the detections of horses, yaks, camels, and cattle into the category of large livestock and those of sheep and goats into small livestock. The subdivision of domestic animals in these two classes is justified by previous research, which found that large and small livestock were managed differently by herders and could therefore affect wild species' spatiotemporal patterns differently (Augugliaro et al., 2020).
We arranged the number of daily detections per species at camera‐trap sites into a matrix of n = 216 sites by S = 14 species. We modeled these detections/nondetections using the joint species distribution model with species correlation and imperfect detection developed by Tobler et al. (2019). This framework is an extension of the multispecies occupancy model (Dorazio & Royle, 2005) that explicitly models the residual correlation in species' occupancy probability (c), corrected for imperfect detection. Our modeling approach is detailed in Appendix S2.
We tested the effect of study area, elevation, terrain slope, and distance from herder settlements on occupancy and the effect of camera‐trap sensitivity and distance from herder settlements on detection probability, at both the community and species levels. The chosen covariates were not correlated according to Spearman's correlation test (|rho| < 0.5). We then calculated the matrix of residual interspecific correlations in occupancy with their corresponding Bayesian credible intervals (BCIs). We implemented the model in a Bayesian framework using JAGS (Plummer, 2003) via R version 3.6.2 (R Development Core Team, 2019) with the R2jags package version 0.5.7–7 (Su & Yajima, 2015). We specified vague prior probabilities for occupancy and detection community hypermeans through normal distributions centered on zero, through uniform priors in an interval from 0 to 10 for the variance of species‐specific random effects, and through uniform distributions in the interval −1 to 1 for the latent variables' regression coefficients (except for the mathematical constraints required for parameter identifiability; see Tobler et al. [2019]). The regression coefficients of large and small livestock were not extracted from the community hypermeans, but were instead treated separately, since their inclusion could have biased the estimation of the response of the wildlife community to covariates. Community hypermeans are therefore to be interpreted as mean effects for the assemblage of wild species only. We generated 3 parallel chains of 200,000 iterations with a burn‐in of 20,000 iterations and thinning by 10 to derive summaries of parameter posterior distribution. We checked for the convergence of the Markov chains based on the Gelman–Rubin statistic (Gelman & Rubin, 1992) and with visual examination of the chains.
To explore the potential relationship between species‐specific body mass and the correlation in occupancy with livestock, we regressed the estimated correlation coefficient of each wild species with the two livestock classes against the log of the species' body mass, which we sourced in Smith et al. (2003) and Hayssen (2008).
We also analyzed the temporal activity pattern of each species using a nonparametric kernel density estimation function implemented with the overlap package (Ridout & Linkie, 2009) in R. We first extracted independent events by aggregating detections separated by less than 30 min and then created the activity distribution curve of each species. To evaluate potential patterns of temporal avoidance or coexistence between the species in the target community, we computed the coefficient of overlap for each pair of species. The coefficient of overlap Δ ranges from 0 (no overlap) to 1 (complete overlap) and is obtained performing pairwise comparisons of diel activity patterns.
RESULTS
Out of the 91 estimated pairwise residual correlation coefficients in occupancy (c), 24 had a 90% BCI that did not overlap zero (Figure 2). Of the correlations in occupancy between wildlife and livestock of both classes, 12 had a 90% BCI that did not overlap zero (Figures 2 and 3), of which 7 were positive (with wolf, red fox, marmot, ground squirrel, and hare) and 5 were negative (with snow leopard, Siberian ibex, and beech marten). Snow leopard, Siberian ibex, and beech marten occupancies were all positively and mutually correlated (snow leopard–Siberian ibex: c = 0.34 [90% BCI: 0.11, 0.52]; snow leopard–beech marten: c = 0.44 [0.21, 0.63]; Siberian ibex–beech marten: c = 0.28 [0.09, 0.45]). Pallas's cat occupancy was positively correlated with marmot (c = 0.44 [0.22, 0.61]) and pika occupancies (c = 0.31 [0.05, 0.54]). Pika occupancy was instead negatively correlated with red fox (c = −0.34 [−0.12, −0.53]), wolverine (c = −0.37 [−0.04, −0.64]), and snow leopard (c = −0.28 [0.05, 0.54]). Red fox was positively correlated with marmot (c = 0.27 [0.08, 0.46]) and wolf (c = 0.29 [0.07, 0.49]). Finally, ground squirrel and marmot were positively correlated (c = 0.40 [0.13, 0.63]).
FIGURE 2.
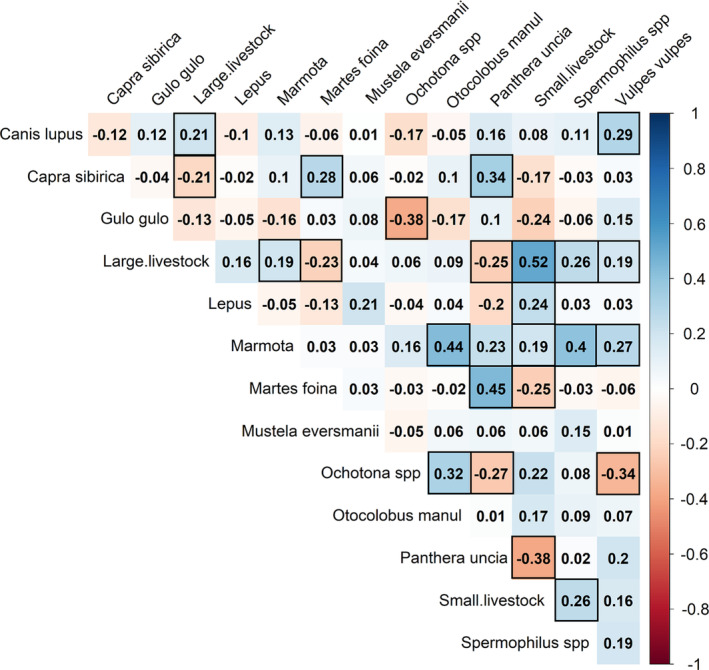
Matrix of all interspecific correlation coefficients (c) in occupancy for wild and domestic mammals detected by camera traps at 216 sites in Mongolian Altai Mountains. Positive correlations are highlighted in blue, negative correlations in red. Species are listed in alphabetical order. Values with a black border are correlation coefficients whose 90% CI did not overlap zero.
FIGURE 3.
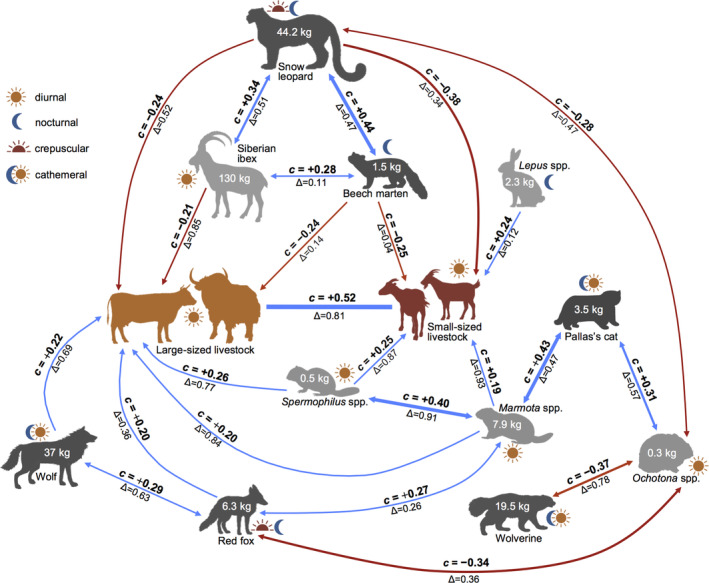
Diagram of statistically strong pairwise spatial and temporal interactions among 11 members of medium‐size to large mammal community and 2 livestock categories (large and small in orange and red, respectively) detected by camera traps in western Mongolia. For each pair of species, the value above the arrow refers to the correlation in occupancy (c), while the one below is the coefficient of overlap (Δ) in activity patterns (see “Data analyses”). Arrows are blue for positive spatial interactions and red for negative ones. The average body mass for each species is also reported, along with the main diel activity pattern (see legend). Carnivores and herbivores are in dark and light gray, respectively.
The correlation in occupancy between wild species and livestock appeared to be negatively related to species body mass, a pattern that was more marked when considering small livestock, as illustrated in Figure 4. Hence, larger wild species tended to have a more negative correlation in occupancy with large and especially small livestock.
FIGURE 4.
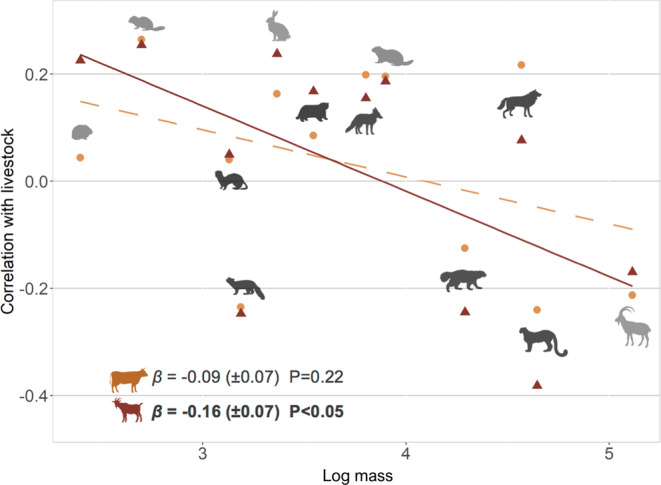
Coefficients of correlation in occupancy (c) between wild species and livestock detected by camera traps in western Mongolia, in relation to the log mass of each species. Orange circles and red triangles indicate the coefficients of correlation with large and small livestock, respectively. Lines show the regressions for large (orange dashed line) and small livestock (red full line). See Figure 3 for names of species depicted.
The analyses of diel activity indicated a clear diurnal pattern for both livestock classes. Among wild species, patterns were highly variable: from clearly nocturnal (Martes foina, Mustela eversmanni, Vulpes vulpes) to fully diurnal (Marmota spp., Ochotona spp., Spermophilus spp.) and more crepuscular species (Panthera uncia) or cathemeral species with irregular activity throughout the day (Canis lupus, Otocolobus manul). All curves are presented in Figure 5. The temporal overlap in activity between livestock and wildlife was low for nocturnal and crepuscular species (in particular Martes foina, Mustela eversmanni, Lepus spp., Panthera uncia), whereas it was high for diurnal species (such as Capra sibirica, Marmota spp., Ochotona spp., Spermophilus spp.). The small and medium‐size rodents and lagomorphs that presented a high degree of temporal overlap with livestock also had a high correlation in occupancy with it. Temporal overlap tended to be intermediate between predators and prey (e.g., Panthera uncia–Capra sibirica Δ = 0.51), whereas it tended to be more pronounced for species in the same trophic guild (e.g., Marmota spp.–Spermophilus spp. Δ = 0.91) (Appendix S3: Figures S1).
FIGURE 5.
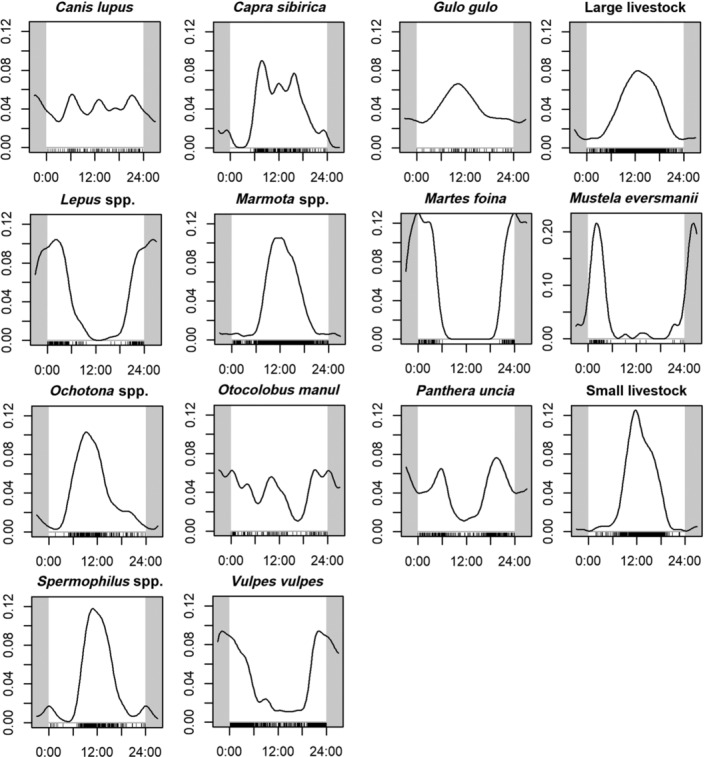
Diel activity patterns of wild and domestic mammals detected by camera traps in western Mongolia. Species are listed alphabetically, with Latin names shown above the subplots. The white box indicates the 24‐h diel cycle, with time of day on the x‐axis and kernel density estimate of activity on the y‐axis.
The mean occupancy estimates for the wildlife community in the four areas were very similar, ranging from 0.35 (0.27–0.43) in Tavan Bogd to 0.38 (0.24–0.53) in Sutai. The regression coefficient for elevation (βelev) on community occupancy was weakly positive, with 90% BCIs that overlapped zero (0.06 [−0.03, 0.16]) and species‐specific estimates that varied from −0.09 [−0.27, 0.07] for the red fox to 0.18 [−0.01, 0.42] for the wolverine Gulo gulo (Appendix S4); predictably, domestic animals and humans were negatively related to elevation (−0.33 [−0.51, −0.16] and −0.51 [−0.71, −0.32] for large and small livestock, respectively). Community occupancy was also lightly related to terrain slope (βslope = 0.07 [−0.01, 0.14]), with relatively consistent species coefficients. In particular, the effects were positive for both Capra sibirica and Otocolobus manul with BCIs that did not overlap zero (0.12 [0.01, 0.26] and 0.12 [0.01, 0.28], respectively). The coefficients for large and small livestock overlapped zero. The community coefficient for distance from settlements (βdis) on occupancy was positive (0.10 [0.02, 0.18]) but again with interspecific variability, with the lowest values for Otocolobus manul, Martes foina, and Ochotona spp. (βdis = 0.08) and highest for Spermophilus spp. (0.13 [0.03, 0.27]; Appendix S4). Predictably, the association was negative for domestic animals, both large (−0.43 [−0.61, −0.25]) and small (−0.53 [−0.75, −0.32]). Finally, community detection probabilities for medium‐ and high‐sensitivity camera‐trap models were largely overlapping (medium sensitivity: βmedium = −3.6 [−4.45, −2.83]; high sensitivity: βhigh = −3.11 [−3.53, −2.69]). Distance from herder settlements was negatively related to community detectability (βdist = −0.15 [−0.32, 0.01]) (Appendix S4).
DISCUSSION
Using camera‐trapping data, activity pattern analysis, and a multispecies occupancy model that incorporates interspecific correlation in occupancy and imperfect detection, we studied the spatiotemporal patterns of occurrence and interspecific relationships within a mammalian community in the Mongolian Altai Mountains.
Each of the 14 species and livestock categories considered showed strong statistical evidence for spatial co‐occurrence (i.e., 90% BCI of c that did not overlap zero) with at least another species, with the only exception being the steppe polecat (Mustela eversmanni). Remarkably, of the 13 interactions that each species can display with any other, the largest number of statistically supported ones involved one of the livestock categories (8 and 6 for large and small livestock, respectively), whereas the highest number of interactions involving wild species was 5, for both snow leopard and marmot. Considering such scores as a simple measure of the magnitude of spatial co‐occurrence among species regardless of their signs, the results supported our choice of including livestock in the analyzed community, given their widespread presence in the region and the potential influence on wildlife (e.g., Berger et al., 2013; Soofi et al., 2018).
Large areas of Mongolia have been subject to seminomadic pastoralism for centuries, but the recent increase in livestock numbers has raised concerns about possible degradation effects (Wesche et al., 2010). Though the numbers of cattle and camels remained relatively constant across the country, a steep increase in sheep and goat populations has been recorded since the early 1990s, with the latter likely representing a greater impact on wildlife than large livestock given their much larger herd size (Hilker et al., 2014). Indeed, we found that the relationship between residual correlation in occupancy of wild and domestic species and wildlife body mass was negative, with a more pronounced effect for the small livestock. This pattern points to a higher sensitivity of larger species to livestock encroachment into protected areas, which is of particular concern for the snow leopard and the Siberian ibex, and more generally mirrors known patterns of size‐dependent vulnerability across mammals (Cardillo et al., 2005; Ripple et al., 2019).
We also found that the probability of site use for the community of wild species increased with increasing distance from permanent sources of human disturbance, mainly herder houses/settlements, which adds evidence for the general influence of livestock herding on co‐occurrence patterns. Notably, such an effect was higher than those of other environmental covariates we considered (elevation and slope). On the one hand, this may be the result of contrasting environmental effects at the species level compensating each other in the mean effect values, or, because species effects are themselves generally weak, it may also reflect the relatively uniform habitat that characterizes the target landscapes. On the other hand, interspecific interactions and anthropogenic disturbance might be more important drivers of species distributions than abiotic variables.
Although, predictably, the correlation in occupancy between large and small livestock was the highest estimated (c = 0.52), it also highlighted differences in site use between the two size classes that support our choice of considering them separately, matching the documented differences in herding practices in the same region and, hence, potential impacts on wildlife (Augugliaro et al., 2020). Notably, the snow leopard showed strong evidence of a negative correlation in occupancy with both livestock categories, with the stronger being with small livestock. At the same time, temporal overlap in diel activity patterns was higher with large livestock, consistent with their being generally free ranging, whereas sheep and goats are held in corrals at night (Augugliaro et al., 2020). The negative correlation in occupancy between Siberian ibex and large livestock (c = −0.21) may indicate a detrimental effect of pastoralism on ibex as a consequence of direct competition for grazing (Mishra et al., 2004; Ripple et al., 2014). Additionally, spatial patterns of interaction among large domestic species and this wild ungulate are possibly accentuated by the high degree of overlap in daily activity patterns (Δ = 0.85). Ibex correlation with small livestock was also negative, though statistically weak, whereas the positive effect of terrain slope on its site use supports the preference of ibex for rugged habitat (Han et al., 2021).
The positive correlation in occupancy between wolf and large livestock (c = 0.22) suggests a role of this carnivore in livestock depredations in the region, especially considering that such a spatial association is not compensated by temporal avoidance (Δ = 0.69). This is in accordance with an earlier study in the same area that involved a focal investigation on predator and prey co‐occurrence (Salvatori et al., 2021). We acknowledge that a high degree of spatiotemporal overlap does not necessarily indicate prey preference, but it does suggest the potential for high encounter rates between carnivores and their prey, which is a key component of prey preference (Allen et al., 2021; Fortin et al., 2015). Conversely, no statistically strong spatial correlation in occupancy was detected for the wolf with herds of small livestock. Overall, the obtained results are consistent with Augugliaro et al. (2020), who found wolves to hunt preferably large rather than small livestock, based on interviews with herders.
Marmots and ground squirrels showed positive correlations with both livestock categories, consistent with previous research reporting that these species have likely benefited from the recent increase in livestock in Mongolia (Gankhuyag et al., 2021). However, this apparent tolerance of burrowing mammals toward livestock is at odds with the documented severe decline of Siberian marmots (Marmota sibirica) in Mongolia in recent decades, possibly related to poaching for the fur market (Batbold, 2002; Kolesnikov et al., 2009), and thus will require further investigation.
As for predator–prey interactions, our results indicated a positive spatial correlation of the snow leopard with its primary wild prey in the region, the Siberian ibex (McCarthy et al., 2016). Similarly, snow leopard site use was positively, although weakly, correlated with that of marmots, which also represent a significant part of its diet (Hacker et al., 2021; Lukarevskiy et al., 2019). Overall, our results are of relevance for this International Union for Conservation of Nature (IUCN)‐Vulnerable top predator for at least three reasons: (1) they provide an indication of spatial displacement of snow leopards by livestock, especially small livestock, which is concerning given the growth of the cashmere market (Berger et al., 2013; e.g., Tumursukh et al., 2016); (2) Siberian ibex appears to be a key driver of occurrence for this carnivore, while there was no strong evidence of an effect of any abiotic covariates on its site use; (3) snow leopards in western Mongolia may prey primarily on wild prey and kill livestock opportunistically, even where the abundance of domestic animals is at least one order of magnitude higher (Augugliaro et al., 2020; Hacker et al., 2021; Johansson et al., 2015; Sharma et al., 2015). These dynamics markedly differ from what was found in other parts of this predator range (Aryal et al., 2014; Bagchi et al., 2004), supporting the notion that the magnitude of human–snow leopard conflicts is highly context‐specific (Snow Leopard Network, 2014). No association emerged instead between wolf and ibex, possibly matching the typical preference of these cursorial predators for structurally less rugged habitat than the one selected by ibex (Suryawanshi et al., 2013). The strong evidence for a positive correlation in occupancy between foxes and marmots (c = 0.27) could mirror both direct predation by the fox on marmots and the use of marmots' galleries as refuges/resting sites by foxes (Murdoch et al., 2016). Similarly, Pallas's cat displayed a remarkably high spatial association with marmots (c = 0.43), whose cavities the cats depend on as denning and resting sites (Ross et al., 2010b), and also a positive association with pikas (c = 0.31), considered the small felid's preferred prey (Ross et al., 2010a).
Concerning intraguild interactions, spatiotemporal overlap between red fox and wolf (c = 0.29, Δ = 0.63) has been documented in other ecological contexts, where wolves attract foxes through increased opportunities to scavenge (Ferretti et al., 2021), a facilitation also observed in relation to other carnivores, such as the Eurasian lynx and the snow leopard (Krofel et al., 2019, 2021). As a complementary result supporting co‐occurrence, both canids showed similar environmental and anthropogenic covariate effects on site use. In contrast, no spatial association or avoidance was observed between the two felids, the snow leopard and Pallas's cat, probably reflecting low dietary niche overlap and infrequent interactions among them. The clear spatiotemporal association between ground squirrels and marmots (c = 0.40, Δ = 0.91) is not surprising given that they belong to the ecosystem engineering group of colonial‐living burrowing animals in grasslands, and they likely present very similar habitat preferences.
As general limitations of our study we acknowledge that (1) the sampling design was aimed at maximizing snow leopard detections, potentially biasing detections of other species and limiting the spatial extent of our inference to the sites sampled; (2) we interpreted occupancy as the probability of site use by the species, given the marked differences among species in home range and movement patterns relative to our sampling design (Neilson et al., 2018) and considering that so‐called true occupancy estimates in continuous habitat depend upon the product between the density of the target species and its home range area (Efford & Dawson, 2012); (3) given the generally high sensitivity of the camera traps we used, the probability of detecting a species was likely largely dictated by its availability for detection (Kéry & Schmidt, 2008), namely, by how often a species passed through a camera's detection zone, given that the camera was located within the home range of one or more individuals of that species; (4) the residual correlations in occupancy we estimated may not entirely reflect actual interactions since they could also be caused by unaccounted‐for differences in habitat use due to missing environmental covariates (Tobler et al., 2019). Though Limitation 1 is unlikely to affect our inference, given that the sites chosen were used by multiple species of the target community, Limitation 2 should be taken into account when interpreting our results. Our sampling design could have led to an overestimation of the proportion of area used by species with large home ranges and high mobility (e.g., snow leopard, wolf, and livestock) while underestimating the proportion of area used by species with small home ranges and short‐distance movements (such as rodents and lagomorphs) (Neilson et al., 2018; Efford & Dawson, 2012). This in turn might have inflated the positive co‐occurrence between species with large home ranges since they have a higher probability of being detected at the same sampling sites, though the home range overlap might, in reality, be limited. Limitation 3 may have minimal effects on our results since the duration of our surveys likely allowed for a good representation of the asymptotic proportion of area used by each species, that is, individuals had enough time to cover a consistent proportion of their home range. Regarding Limitation 4, we cannot rule out the possibility that the inclusion of other abiotic covariates in our model would change the output for the biotic relationships. However, the target ecosystem is characterized by low vegetation diversity, and most environmental variability derives from topography, which we accounted for by including elevation and terrain slope in our model.
In conclusion, systematic camera trapping proved a valuable tool in studying the interspecific relationships within a community of wild and domestic mammals inhabiting remote and hardly accessible mountain landscapes. In line with our predictions, livestock encroaching into protected areas influenced the whole community of wild mammals, with their average probability of occurrence increasing away from herder settlements and a predominant number of potential spatial interactions involving livestock. The sign of the co‐occurrence of livestock and wildlife was species‐dependent, with larger species generally showing a tendency to avoid livestock (with the remarkable exception of the wolf that often prey on it) and rodents and lagomorphs tending to positively co‐occur with it. As predicted, predators and their focal prey generally had positive correlation in occupancy, whereas, contrary to our expectations, a number of intraguild species pairs also showed positive co‐occurrence, with no evidence of spatial or temporal niche partitioning; further, focal investigations are needed to clarify whether this pattern is caused by the use of similar resources by species of the same guild. Though pastoralism has coexisted with wildlife for millennia in Central Asian grasslands, our findings suggest that the recent dramatic increase in livestock numbers calls for new policies and strategies to decrease the pressure of livestock husbandry on wildlife communities, with special attention on large wild mammals, namely, snow leopard and Siberian ibex, which seem to be potentially more sensitive than smaller mammals to the pervasive presence of domestic animals.
AUTHOR CONTRIBUTIONS
Francesco Rovero and Claudio Augugliaro conceptualized the study and secured funding. Francesco Rovero and Valentina Oberosler designed the field work. Francesco Rovero, Valentina Oberosler, and Miha Krofel collected the data. Marco Salvatori, Francesco Rovero, and Valentina Oberosler conducted data screening, preparation, and analyses. Valentina Oberosler and Marco Salvatori created the figures. Marco Salvatori, Valentina Oberosler, and Francesco Rovero wrote the paper with contributions from all authors.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
ACKNOWLEDGMENTS
We thank Al S. Glen and an anonymous reviewer for their useful advice that helped improve this manuscript. Funding for fieldwork came from Panthera Sabin Snow Leopard grants, Arca Foundation–Parco Natura Viva, Wildlife Initiative, Irbis Mongolia and the Altai Institute for Research and Conservation. The Gino Zobele Fund supported Valentina Oberosler's contribution, the Herbette Foundation from the University of Lausanne supported Claudio Augugliaro's contribution, and Slovenian Research Agency (Grants N1‐0163 and P4‐0059) supported Miha Krofel's contribution. The Snow Leopard Conservancy and the Fototrappolaggio company provided part of the camera traps. We are grateful to the Mongolian Ministry of Environment and Tourism for research permits and support and to Choikhand Janchivlamdan of Green Initiative and Munkhtsog Bariusha of the Mongolian Academy of Science for logistic support. We thank Asja Marchetto and Silvia Palmarini for the invaluable help in the identification of photographed animals. We also thank the following colleagues, field assistants, translators, and rangers for their invaluable help during fieldwork: Rasmus Havmøller, Renato Rizzoli, Ibra Monti, Paolo Zorer, Barry Rosenbaum, Jukhan Mengerbay, Elik Nurbat, Jankei Khuantkan, Huandag Baymanday, Dario Ciaramella, B. Barsuren, B. Munkh‐Erdene, and T. Yondonjamts. Marco Salvatori thanks Sofia Farina for help with the mathematical formulation of the model. Open Access Funding provided by Universita degli Studi di Firenze within the CRUI‐CARE Agreement.
Salvatori, Marco , Oberosler Valentina, Augugliaro Claudio, Krofel Miha, and Rovero Francesco. 2022. “Effects of Free‐Ranging Livestock on Occurrence and Interspecific Interactions of a Mammalian Community.” Ecological Applications 32(7): e2644. 10.1002/eap.2644
Handling Editor: Neil H. Carter
Funding information Arca Foundation; Mongolian Academy of Science; Mongolian Ministry of Environment and Tourism; Parco Natura Viva, Wildlife Initiative; Slovenian Research Agency, Grant/Award Numbers: P4‐0059, N1‐0163; University of Lausanne
DATA AVAILABILITY STATEMENT
Data (Salvatori et al., 2022) are provided in Figshare at https://doi.org/10.6084/m9.figshare.19314176.
REFERENCES
- Allen, M. L. , Sibarani M. C., and Krofel M.. 2021. “Predicting Preferred Prey of Sumatran Tigers Panthera tigris sumatrae Via Spatio‐Temporal Overlap.” Oryx 55(2): 197–203. [Google Scholar]
- Aryal, A. , Brunton D., Ji W., Barraclough R. K., and Raubenheimer D.. 2014. “Human–Carnivore Conflict: Ecological and Economical Sustainability of Predation on Livestock by Snow Leopard and Other Carnivores in the Himalaya.” Sustainability Science 9(3): 321–9. [Google Scholar]
- Augugliaro, C. , Christe P., Janchivlamdan C., Baymanday H., and Zimmermann F.. 2020. “Patterns of Human Interaction with Snow Leopard and Co‐Predators in the Mongolian Western Altai: Current Issues and Perspectives.” Global Ecology and Conservation 24: e01378. [Google Scholar]
- Bagchi, S. , Mishra C., and Bhatnagar Y. V.. 2004. “Conflicts between Traditional Pastoralism and Conservation of Himalayan Ibex (Capra sibirica) in the Trans‐Himalayan Mountains.” Animal Conservation 7(2): 121–8. [Google Scholar]
- Bagchi, S. , Namgail T., and Ritchie M. E.. 2006. “Small Mammalian Herbivores as Mediators of Plant Community Dynamics in the High‐Altitude Arid Rangelands of Trans‐Himalaya.” Biological Conservation 127(4): 438–42. [Google Scholar]
- Batbold, J. 2002. “The Problems of Management of Marmots in Mongolia.” In Holarctic Marmots as a Factor of Biodiversity, Proceedings of the Third International Conference on Marmots, Cheboksary, Russia, edited by Armitage K. B. and Rumiantsev V. Y., 68–75. Moscow: ABF Publishing House. [Google Scholar]
- Berger, J. , Buuveibaatar B., and Mishra C.. 2013. “Globalization of the Cashmere Market and the Decline of Large Mammals in Central Asia.” Conservation Biology 27(4): 679–89. [DOI] [PubMed] [Google Scholar]
- Brook, L. A. , Johnson C. N., and Ritchie E. G.. 2012. “Effects of Predator Control on Behaviour of an Apex Predator and Indirect Consequences for Mesopredator Suppression.” Journal of Applied Ecology 49(6): 1278–86. [Google Scholar]
- Cardillo, M. , Mace G. M., Jones K. E., Bielby J., Bininda‐Emonds O. R., Sechrest W., et al. 2005. “Multiple Causes of High Extinction Risk in Large Mammal Species.” Science 309(5738): 1239–41. [DOI] [PubMed] [Google Scholar]
- Chetri, M. , Odden M., and Wegge P.. 2017. “Snow Leopard and Himalayan Wolf: Food Habits and Prey Selection in the Central Himalayas, Nepal.” PLoS One 12(2): e0170549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, C. L. , Rich L. N., Farris Z. J., Kelly M. J., di Bitetti M. S., Blanco Y. D., et al. 2018. “Ecological Correlates of the Spatial Co‐Occurrence of Sympatric Mammalian Carnivores Worldwide.” Ecology Letters 21(9): 1401–12. [DOI] [PubMed] [Google Scholar]
- Dorazio, R. M. , and Royle J. A.. 2005. “Estimating Size and Composition of Biological Communities by Modeling the Occurrence of Species.” Journal of the American Statistical Association 100(470): 389–98. [Google Scholar]
- Efford, M. G. , and Dawson D. K.. 2012. “Occupancy in Continuous Habitat.” Ecosphere 3(4): 1–15. [Google Scholar]
- Ekernas, L. S. , Sarmento W. M., Davie H. S., Reading R. P., Murdoch J., Wingard G. J., Amgalanbaatar S., and Berger J.. 2017. “Desert pastoralists' Negative and Positive Effects on Rare Wildlife in the Gobi.” Conservation Biology 31(2): 269–77. [DOI] [PubMed] [Google Scholar]
- Fegraus, E. , and MacCharty J.. 2016. “Camera Trap Data Management and Interoperability.” In Camera Trapping for Wildlife Research, edited by Rovero F. and Zimmermann F., 33–42. Exeter: Pelagic Publisher, UK. [Google Scholar]
- Feng, J. , Sun Y., Li H., Xiao Y., Zhang D., Smith J. L., et al. 2021. “Assessing Mammal Species Richness and Occupancy in a Northeast Asian Temperate Forest Shared by Cattle.” Diversity and Distributions 27(5): 857–72. [Google Scholar]
- Ferretti, F. , Pacini G., Belardi I., Sensi M., de Oliveira R., et al. 2021. “Recolonizing Wolves and Opportunistic Foxes: Interference or Facilitation?” Biological Journal of the Linnean Society 132(1): 196–210. [Google Scholar]
- Fortin, D. , Buono P. L., Schmitz O. J., Courbin N., Losier C., St‐Laurent M. H., Drapeau P., et al. 2015. “A Spatial Theory for Characterizing Predator–Multiprey Interactions in Heterogeneous Landscapes.” Proceedings of the Royal Society B: Biological Sciences 282(1812): 20150973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gankhuyag, G. , Ceacero F., Yansanjav A., Hejcmanová P., Davaa L., Namkhaidorj S., and Bolfíková B. Č.. 2021. “Long‐Term Trends in Livestock and Wildlife Interactions: Do Livestock Numbers Predict Recent Trends of Wolves, Foxes, and rodents' Populations in Mongolian Rangelands?” Journal for Nature Conservation 60: 125969. [Google Scholar]
- Gelman, A. , and Rubin D. B.. 1992. “A Single Series from the Gibbs Sampler Provides a False Sense of Security.” Bayesian Statistics 4: 625–31. [Google Scholar]
- Hacker, C. E. , Jevit M., Hussain S., Muhammad G., Munkhtsog B., Munkhtsog B., Zhang Y., et al. 2021. “Regional Comparison of Snow Leopard (Panthera Uncia) Diet Using DNA Metabarcoding.” Biodiversity and Conservation 30: 797–817. [Google Scholar]
- Han, L. , Wang Z., Blank D., Wang M., and Yang W.. 2021. “Different Environmental Requirements of Female and Male Siberian Ibex, Capra sibirica .” Scientific Reports 11(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayssen, V. 2008. “Patterns of Body and Tail Length and Body Mass in Sciuridae.” Journal of Mammalogy 89(4): 852–73. [Google Scholar]
- Hebblewhite, M. , White C. A., Nietvelt C. G., McKenzie J. A., Hurd T. E., Fryxell J. M., Bayley S. E., and Paquet P. C.. 2005. “Human Activity Mediates a Trophic Cascade Caused by Wolves.” Ecology 86(8): 2135–44. [Google Scholar]
- Hilker, T. , Natsagdorj E., Waring R. H., Lyapustin A., and Wang Y.. 2014. “Satellite Observed Widespread Decline in Mongolian Grasslands Largely Due to Overgrazing.” Global Change Biology 20(2): 418–28. [DOI] [PubMed] [Google Scholar]
- Hoekstra, J. M. , Boucher T. M., Ricketts T. H., and Roberts C.. 2005. “Confronting a Biome Crisis: Global Disparities of Habitat Loss and Protection.” Ecology Letters 8(1): 23–9. [Google Scholar]
- Jackson, R. M. , Roe J. D., Wangchuk R., and Hunter D. O.. 2005. “Camera‐Trapping of Snow Leopards.” Cat News 42: 19. [Google Scholar]
- Johansson, Ö. , McCarthy T., Samelius G., Andrén H., Tumursukh L., and Mishra C.. 2015. “Snow Leopard Predation in a Livestock Dominated Landscape in Mongolia.” Biological Conservation 184: 251–8. [Google Scholar]
- Kéry, M. , and Schmidt B.. 2008. “Imperfect Detection and its Consequences for Monitoring for Conservation.” Community Ecology 9(2): 207–16. [Google Scholar]
- Kiffner, C. , Wenner C., LaViolet A., Yeh K., and Kioko J.. 2015. “From Savannah to Farmland: Effects of Land Use on Mammal Communities in the Tarangire–Manyara Ecosystem, Tanzania.” African Journal of Ecology 53: 156–66. [Google Scholar]
- Kolesnikov, V. V. , Brandler O. V., Badmaev B. B., Zoje D., and Adiya Y.. 2009. “Factors that Lead to a Decline in Numbers of Mongolian Marmot Populations.” Ethology Ecology and Evolution 21(3–4): 371–9. [Google Scholar]
- Krofel, M. , Groff C., Oberosler V., Augugliaro C., and Rovero F.. 2021. “Snow Leopard (Panthera uncia) Predation and Consumption of an Adult Yak in the Mongolian Altai.” Ethology, Ecology and Evolution 33(6): 636–43. [Google Scholar]
- Krofel, M. , Skrbinšek T., and Mohorović M.. 2019. “Using Video Surveillance to Monitor Feeding Behaviour and Kleptoparasitism at Eurasian Lynx Kill Sites.” Journal of Vertebrate Biology 68(4): 274–84. [Google Scholar]
- Lukarevskiy, V. S. , Dalannast M., Lukarevskiy S., and Damdin E.. 2019. “Factors Determining the Distribution and Status of the Snow Leopard Population (Panthera uncia) in Western Mongolia.” Animal and Veterinary Sciences 7(6): 127–32. [Google Scholar]
- McCarthy, T. , Mallon D., Sanderson E. W., Zahler P., and Fisher K.. 2016. “What is a snow leopard? Biogeography and Status Overview.” In Snow Leopards 23–42. Academic Press, London, UK. [Google Scholar]
- Mishra, C. , van Wieren S. E., Ketner P., Heitkonig I. M. A., and Prins H. H. T.. 2004. “Competition between Domestic Livestock and Wild Bharal Pseudois nayaur in the Indian Trans‐Himalaya.” Journal of Applied Ecology 41: 344–54. [Google Scholar]
- Mueller, T. , Olson K. A., Fuller T. K., Schaller G. B., Murray M. G., and Leimgruber P.. 2008. “In Search of Forage: Predicting Dynamic Habitats of Mongolian Gazelles Using Satellite‐Based Estimates of Vegetation Productivity.” Journal of Applied Ecology 45(2): 649–58. [Google Scholar]
- Murdoch, J. D. , Davie H., Galbadrah M., and Reading R. P.. 2016. “Factors Influencing Red Fox Occupancy Probability in Central Mongolia.” Mammalian Biology 81(1): 82–8. National Statistics Office of Mongolia (2019). https://www.en.nso.mn/ [Google Scholar]
- Neilson, E. W. , Avgar T., Burton A. C., Broadley K., and Boutin S.. 2018. “Animal Movement Affects Interpretation of Occupancy Models from Camera‐Trap Surveys of Unmarked Animals.” Ecosphere 9(1): e02092. [Google Scholar]
- Newsome, T. M. , Greenville A. C., Ćirović D., Dickman C. R., Johnson C. N., Krofel M., Letnic M., et al. 2017. “Top Predators Constrain Mesopredator Distributions.” Nature Communications 8: 15469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez, S. , Alkemade R., Kok K., and Leemans R.. 2020. “Potential Biodiversity Change in Central Asian Grasslands: Scenarios for the Impact of Climate and Land‐Use Change.” Regional Environmental Change 20(2): 1–13. [Google Scholar]
- Plummer M. 2003. “JAGS: A Program for Analysis of Bayesian Graphical Models Using Gibbs Sampling.” Proceedings of the 3rd International Workshop on Distributed Statistical Computing, Vienna, Austria.
- QGIS Development Team . 2018. “QGIS Geographic Information System.” Open Source Geospatial Foundation Project. http://qgis.osgeo.org/.
- R Core Team . 2019. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; https://www.R-project.org/. [Google Scholar]
- Richmond, O. M. , Hines J. E., and Beissinger S. R.. 2010. “Two‐Species Occupancy Models: A New Parameterization Applied to Co‐Occurrence of Secretive Rails.” Ecological Applications 20(7): 2036–46. [DOI] [PubMed] [Google Scholar]
- Richter, A. , and Osborne W.. 2014. “Temperate Grasslands: Out of Sight, out of Mind? Conservation and Research Priorities for One of the world's most Threatened Ecological Communities.” In Grassland Biodiversity and Conservation in a Changing World 1–26. New York: Nova Publishers. [Google Scholar]
- Ridout, M. , and Linkie M.. 2009. “Estimating Overlap of Daily Activity Patterns from Camera Trap Data.” Journal of Agricultural, Biological, and Environmental Statistics 14(3): 322–37. [Google Scholar]
- Ripple, W. J. , Estes J. A., Beschta R. L., Wilmers C. C., Ritchie E. G., Hebblewhite M., Berger J., et al. 2014. “Status and Ecological Effects of the World's Largest Carnivores.” Science 343: 6167. [DOI] [PubMed] [Google Scholar]
- Ripple, W. J. , Wolf C., Newsome T. M., Betts M. G., Ceballos G., Courchamp F., Hayward M. W., Valkenburgh B., Wallach A. D., and Worm B.. 2019. “Are We Eating the World's Megafauna to Extinction?” Conservation Letters 12(3): e12627. [Google Scholar]
- Ross, S. , Kamnitzer R., Munkhtsog B., and Harris S.. 2010b. “Den‐Site Selection Is Critical for Pallas's Cats (Otocolobus manul).” Canadian Journal of Zoology 88: 905–13. [Google Scholar]
- Ross, S. , Munkhtsog B., and Harris S.. 2010a. “Dietary Composition, Plasticity, and Prey Selection of Pallas's Cats.” Journal of Mammalogy 91(4): 811–7. [Google Scholar]
- Rottstock, T. , Göttert T., and Zeller U.. 2020. “Relatively Undisturbed African Savannas‐an Important Reference for Assessing Wildlife Responses to Livestock Grazing Systems in European Rangelands.” Global Ecology and Conservation 23: e01124. [Google Scholar]
- Rovero, F. , Augugliaro C., Havmøller R. W., Groff C., Zimmermann F., Oberosler V., and Tenan S.. 2020. “Co‐Occurrence of Snow Leopard Panthera Uncia, Siberian Ibex Capra sibirica and Livestock: Potential Relationships and Effects.” Oryx 54(1): 118–24. [Google Scholar]
- Salvatori, M. , Oberosler V., Augugliaro C., Krofel M., and Rovero F.. 2022. “Effects of Free‐Ranging Livestock on Occurrence and Interspecific Interactions of a Mammalian Community.” Figshare, dataset. 10.6084/m9.figshare.19314176.v1. [DOI] [PMC free article] [PubMed]
- Salvatori, M. , Tenan S., Oberosler V., Augugliaro C., Christe P., Groff C., Krofel M., Zimmermann F., and Rovero F.. 2021. “Co‐Occurrence of Snow Leopard, Wolf and Siberian Ibex under Livestock Encroachment into Protected Areas across the Mongolian Altai.” Biological Conservation 261: 109294. [Google Scholar]
- Sawamukai, M. , Hoshino B., Ganzorig S., Purevsuren T., Asakawa M., and Kawashima K.. 2012. “Preliminary Results on Surface and Soil Characteristics of Brandt's Vole (Microtus brandti) Habitat in Central Mongolia Using Satellite Data.” Journal of Arid Land Studies 22: 295–8. [Google Scholar]
- Sharma, R. K. , Bhatnagar Y. V., and Mishra C.. 2015. “Does Livestock Benefit or Harm Snow Leopards?” Biological Conservation 190: 8–13. [Google Scholar]
- Smith, F. A. , Lyons S. K., Ernest S. M., Jones K. E., Kaufman D. M., Dayan T., et al. 2003. “Body Mass of Late Quaternary Mammals: Ecological Archives E084‐094.” Ecology 84(12): 3403–3. [Google Scholar]
- Snow Leopard Network . 2014. Snow Leopard Survival Strategy. Revised 2014 Version. Seattle, WA: Snow Leopard Network. [Google Scholar]
- Soofi, M. , Ghoddousi A., Zeppenfeld T., Shokri S., Soufi M., Jafari A., Ahmadpour M., et al. 2018. “Livestock Grazing in Protected Areas and its Effects on Large Mammals in the Hyrcanian Forest, Iran.” Biological Conservation 217: 377–82. [Google Scholar]
- Su Y‐S, and Yajima M.. 2015. “R2jags using R to run JAGS.” https://rdrr.io/cran/R2jags/
- Suraci, J. P. , Gaynor K. M., Allen M. L., Alexander P., Brashares J. S., Cendejas Zarelli S., et al. 2021. “Disturbance Type and Species Life History Predict Mammal Responses to Humans.” Global Change Biology 27: 3718–31. [DOI] [PubMed] [Google Scholar]
- Suryawanshi, K. R. , Bhatnagar Y. V., Redpath S., and Mishra C.. 2013. “People, Predators and Perceptions: Patterns of Livestock Depredation by Snow Leopards and Wolves.” Journal of Applied Ecology 50(3): 550–60. [Google Scholar]
- Tobler, M. W. , Kéry M., Hui F. K., Guillera‐Arroita G., Knaus P., and Sattler T.. 2019. “Joint Species Distribution Models with Species Correlations and Imperfect Detection.” Ecology 100(8): e02754. [DOI] [PubMed] [Google Scholar]
- Tumursukh, L. , Suryawanshi K. R., Mishra C., McCarthy T. M., and Boldgiv B.. 2016. “Status of the Mountain Ungulate Prey of the Endangered Snow Leopard Panthera uncia in the Tost Local Protected Area, South Gobi, Mongolia.” Oryx 50(2): 214–9. [Google Scholar]
- Venter, O. , Sanderson E. W., Magrach A., Allan J. R., Beher J., Jones K. R., Possingham H. P., et al. 2016. “Sixteen Years of Change in the Global Terrestrial Human Footprint and Implications for Biodiversity Conservation.” Nature Communications 7(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche, K. , Ronnenberg K., Retzer V., and Miehe G.. 2010. “Effects of Large Herbivore Exclusion on Southern Mongolian Desert Steppes.” Acta Oecologica 36(2): 234–41. [Google Scholar]
- Wisz, M. S. , Pottier J., Kissling W. D., Pellissier L., Lenoir J., Damgaard C. F., Dormann C. F., et al. 2013. “The Role of Biotic Interactions in Shaping Distributions and Realised Assemblages of Species: Implications for Species Distribution Modeling.” Biological Reviews 88(1): 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Data Availability Statement
Data (Salvatori et al., 2022) are provided in Figshare at https://doi.org/10.6084/m9.figshare.19314176.


