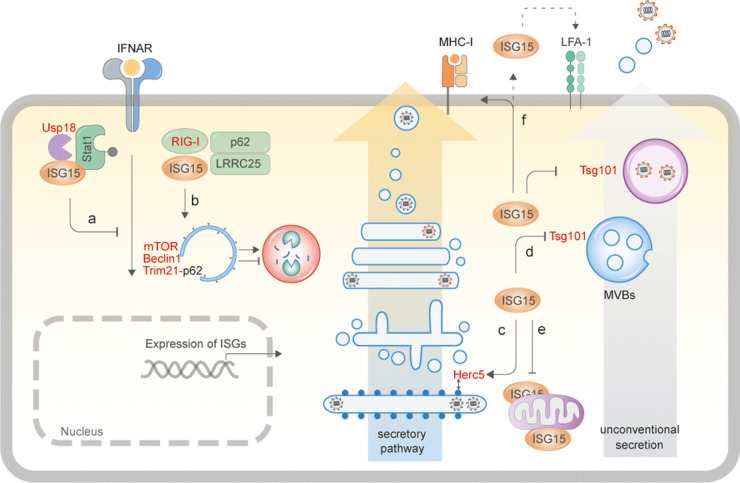
Figure 2. ISG15-driven cellular responses.
(a) Human ISG15 binds to Usp18 and prevents its ubiquitylation and proteasomal degradation [17,55]. Usp18 in turn binds to Stat2 and down-regulates IFNAR2, thereby suppressing IFN-signalling. In the absence of ISG15, Usp18 is degraded, resulting in prolonged IFN-I signalling and inflammatory responses [17,55]. (b) ISG15 binds to RIG-I-LRRC25-p62 to induce their autophagosomal degradation and prevent excessive immune activation [52], and has also been shown to trigger autophagy by modifying mTOR in HEK293T cells [54], but equally is able to block autophagic flux by modifying Beclin1 [49] and Trim21 [51]. (c) ISG15 modifies newly synthesised secretory proteins via Herc5 association with polyribosomes [35]. In infected cells, this phenomenon results primarily in ISGylation of viral capsids leading to inhibition of virus assembly. ISGylation of proteins of the secretory pathway can potentially increase secretion of cytokines [42] and MHC-I presentation [43]. (d) ISGylation of components of the multivesicular body results in inhibition of exosomal release, and transport and secretion of virus proteins/particles via unconventional secretory processes [32,41]. (e) ISGylation of mitochondrial components can result in dysregulation of metabolic processes [53,74]. (f) Monomeric, free ISG15 can be secreted out of cells [69] to function as a cytokine by binding to LFA-1 expressing cells [85]. Verified interaction or modification of proteins by ISG15 is depicted in red.