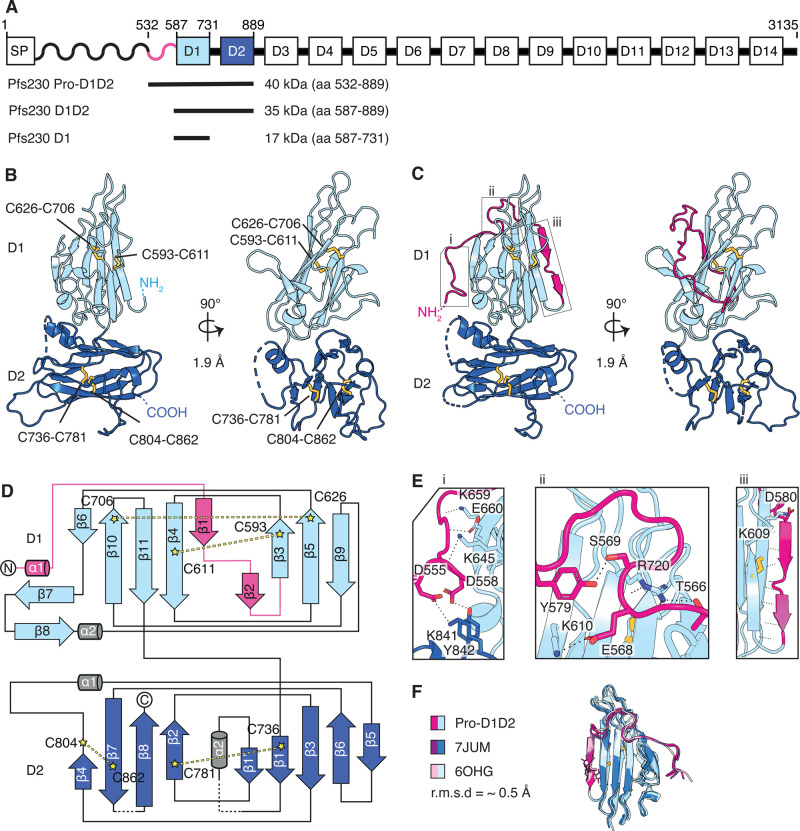
Figure 1. Crystal structures of Pfs230 D1D2 in the presence and absence of pro-domain residues.
(A) Schematic diagram of Pfs230 and recombinant protein constructs. Signal peptide (SP), prodomain (wave) and 14 6-cys domains denoted from D1–D14 are indicated. (B) Crystal structure of Pfs230 D1D2 in two orthogonal views. N- and C-termini and disulfide bonds (yellow) are indicated. The D1 domain is shown in light blue and D2 domain in dark blue. Disulfide bonds are shown in ball and stick representation. (C) Crystal structure of Pfs230 Pro-D1D2 in two orthogonal views. Prodomain residues are shown in pink. Disulfide bonds (yellow) are shown in ball and stick representation. (D) Topology diagram of Pfs230 Pro-D1D2 is colored similarly to the schematic representation in Figure 1C. The disulfide bonds C593–C611 and C626–C706 are indicated in D1, and disulfide bonds C736–C781 and C804–C862 in D2. (E) Interactions between prodomain residues (pink) with residues of the 6-cys domains D1 and D2. Panels i to iii refer to the corresponding regions in Figure 1C. (F) Alignment of the D1 domain of Pfs230 Pro-D1D2 with Pfs230 of PDB ID 6OGH and 7JUM.