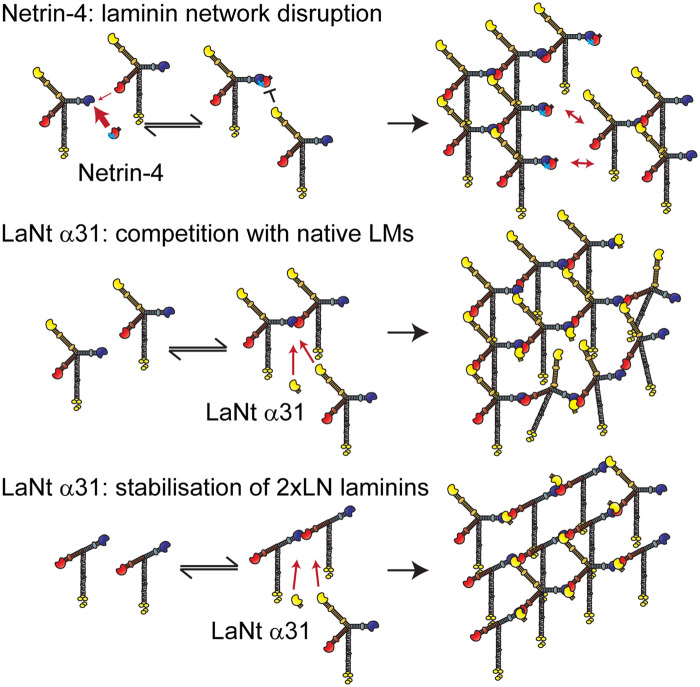
Figure 3. Netrin-4 and LaNt α31 predicted effects on laminin networks.
(Top) Netrin-4 binding with high affinity to laminin γ-chains can outcompete the laminin β-chains. However, α-chains cannot bind to γ/netrin-4 complexes. Therefore, netrin-4 disrupts laminin networks leading to locally increased pore size and decreased basement membrane stiffness. (Middle) LaNt α31 has an identical laminin N-terminal domain as LMα3b and therefore LaNt α31 will compete with approximately equal affinity for α-chain binding sites. This will lead to a partially disrupted network with reduced stiffness, with the level of disruption proportional to the expression level. (Bottom) In basement membranes containing T-shaped laminins, where only the β- and γ-chains contain N-terminal domains (e.g. LM411), the LaNt α31 protein may stabilise transient βγ interactions allowing the formation of stable ternary nodes. This would lead to linear arrays of the two-arm laminins. These arrays could also be cross-linked via the integration of some three-arm laminins within the local structure. The mechanical properties of the formed network would depend on the ratio of the three-arm to two-arm laminins and the local LaNt α31 concentrations.