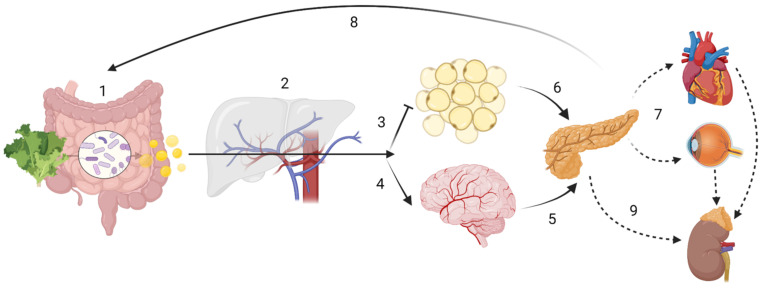
Figure 1.
Host-driven variability in SCFA metabolism and distribution may lead to different disease outcomes. ADME (sub-) steps may explain the variability in SCFA effects. The enterotype influences the amount of SCFA produced, while human digestive enzymatic activity may regulate microbial communities; (1) Absorption: SNPs in mucin, MCTs or tight junction function could impair SCFA bioavailability. Butyrate is the main energy source for colonocytes. (2) In the portal circulation SCFA undergo first-pass effects, where a majority of propionate is metabolized via GPR109A, GPR43 and GPR41, having gluconeogenic or lipogenic effects. Distribution In the systemic circulation: although at present at low concentrations, butyrate and propionate are still detectable; acetate is now the most abundant SCFA. (3) Acetate inhibits lipolysis at the adipose tissue level. (4) Acetate can cross the “blood-brain-barrier” (BBB). Metabolism: SCFA have showed to be effective against microglial oxidative stress responses. SCFA may also have cellular signalling properties, as evidenced by its control of centrally released insulin (6) or its impact on the hypothalamic-pituitary-adrenal axis in leptin and cortisol responses, which may ultimately lead into maladaptive health conditions across the body (7). Finally, gluconeogenic, lipogenic and insulinogenic signals impact ghrelin, leptin and peptide YY release, leading to appetite suppression and satiety (8), improved insulin sensitivity and glucose metabolism, as well as reduction of serum lipids. (9) Excretion: in the kidney, SCFA can be re-absorbed by MCT1. Note: the intracellular effect of SCFA e.g., on HDAC or NF-κB are not displayed. Created with BioRender.com.